This is a material that is one atom thick and has applications in nanoelectronics, energy storage and as a future component in vehicles and airplanes
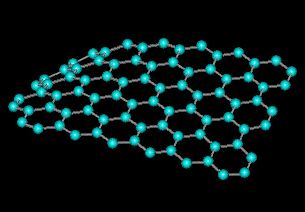
A new graphene-based material, which helps decipher the structure of graphite oxide and could lead to other possible discoveries regarding the one-atom-thick material known as graphene, which has applications in nanoelectronics, energy storage and as a future component in vehicles and airplanes, was synthesized by researchers from the University of Texas.
In order to get an idea about the graphene nanomaterial, we have to imagine a light-weight material with the strongest chemical bonds known in nature, and consequently with extraordinary mechanical properties. In addition, it conducts heat better than any other material and contains charge carriers that pass through it at enormous speed. Graphene, which is only one atom thick, consists of a set of bonds of carbon atoms in the structure of a wire with a hexagonal mesh (chicken wire) like a honeycomb and is actually a single layer of graphite.
Mechanical engineering professor Rod Ruoff and his publishing partners succeeded, for the first time, in labeling graphite with a magnetically active carbon atom (C13). They first made graphite with normal carbon atoms (C12) that are not magnetically active, then replaced them with active carbon atoms and oxidized the graphite to get its oxide. At this stage they used solid-state nuclear magnetic resonance (NMR) to discern the detailed chemical structure of graphite oxide. The results of these experiments appear in the scientific journal Science.
"As a result of our publication, scientists and engineers will now be able to produce different types of graphene (using carbon-13-labeled graphene as the starting material on which known chemistry will be carried out) and study such graphene-based materials using solid-state nuclear magnetic resonance to obtain their detailed chemical structure," says the principal investigator. "This includes situations such as in cases where graphene is mixed with a polymer and chemically bonded at critical points for the preparation of composite materials; or embedded in ceramic materials or glass; or its use in nanoelectronic components; Or mix it with electrolyte to get more efficient batteries. If we don't know the detailed chemistry of these materials, we won't be able to improve their properties."
Graphene-based materials are a research area that is at the center of attention in universities because they are expected to have applications for extremely strong and at the same time light-weight materials that could be used in vehicles and airplanes while improving the efficiency of fuel cells, in wind turbine blades for improved production of electrical energy, as essential components in nano Electronics that will be much more efficient, for storing electrical energy in batteries, and in transparent conductive fibers that will be used in solar cells and digital displays. In each of these applications, subtle chemical interactions with external materials will play a significant role in understanding and optimizing their performance.
Ruoff and his team proved that they had indeed produced this material with the help of researcher Frank Stadermann of the University of Washington. The researcher used a special mass spectrometer that is normally used to measure the distribution of isotopes of various elements present in micrometeorites that have landed on the Earth's surface (a mass spectrometer is a device that measures the ratio between the charge and the mass of charged particles. Through this measurement, a sample can be analyzed, and the composition of the materials can be determined she returned.
This device is of great importance in analytical chemistry, and is used to identify substances in a variety of applications, including forensics, industrial quality assurance, and is used for research in broad fields: nuclear physics, chemistry, biology, archeology, etc.). Then one hundred percent of the graphite labeled with carbon-13 became one hundred percent graphite oxide also labeled with carbon-13. This oxide also exists as a multi-layer material, but it also contains oxygen atoms linked to graphene through chemical bonds. In the next step, the researchers used the solid state GNP method to discover the full details of the oxide structure. The lead researcher explains that although this oxide was synthesized about one hundred and fifty years ago, the exact location of the oxygen atoms was subject to scientific controversy, even recently.
"The ability to control the isotopic labeling between carbon-12 and carbon-13 will certainly lead to a multitude of studies of different types," notes the lead researcher.
The press release from the University of Texas
More of the topic in Hayadan:

3 תגובות
Here it is written that they used solid-state nuclear magnetic resonance (NMR) to discern the detailed chemical structure of graphite oxide.
As far as I know, X-ray crystallography is also used to determine the chemical structure of molecules.
When do you use this or that procedure? Or are these two different things?
The last paragraph "This device is owned by..." etc. is clearly incomprehensible.
At the same time, the explanation about the device should be praised and the science writers should learn to expand and enrich the knowledge of the readers who are thirsty for information and science. Instruments are a very interesting part of science and I'm sure many readers would be happy to know much more about them than the writers volunteer.
What exactly is meant by "magnetically active"? After all, the number of electrons in both types of isotopes is the same. Does their arrangement in the orbitals change due to an additional neutron?