In short: researchers created More Tiny robots, which in the future may help heal wounds, restore tissues and even treat diseases. The interesting innovation is that the robots are made of human cells, which joined together by themselves (with a slight drive) and developed means of self-propulsion
Two years ago some scientists decided to do the unbelievable, or at least the very strange. They took frog embryos and dissected them into their individual cells. They then painstakingly worked with the finest tweezers they could find to reattach the cells to each other in a pattern recommended to them by an advanced artificial intelligence. When the frog cells were reconnected together in this way, they together created a tiny robot - the size of less than a dot on the page - which was named a 'xenobot'. Why Xenobot? Based on the scientific name of the frog - xenopus, Xenopus in Hebrew.
The xenobots were not called 'robot' for nothing. They managed to perform impressive tasks in the petri dish: they moved around by themselves in the solution and found their way in unfamiliar paths. They could heal themselves after injury, collect and carry other cells from the environment, and even replicate themselves for several generations.
It was an impressive success at the time, and when I wrote about it at the time, I predicted that those xenobots might still perform treatments inside the human body. For example, to locate cancer cells in the bloodstream and destroy them. The problem is that no one wants robots made up of frog cells inside the body. Not necessarily because of the element of disgust, but because such cells may trigger an acute immune response from the body. For this reason it would have been better to use human cells to build the xenobots. But is it even possible to do this? Or maybe only frog cells are able to reconnect to new forms that nature has not dreamed of?
And here, at the end of 2023, the original researchers proved to us that it is possible to perform the same thing - roughly - also with human cells. And unlike the frog cells, which came from frog embryos, this time we are talking about mature cells found in the body of each of us. It turns out that these can also reconnect and assemble robots. But what do we call them? These are not frog cells!
That's why the new robots were named anthropots, from the word "anthro" (human) and bots (not on Twitter).
How was an anthropot born?
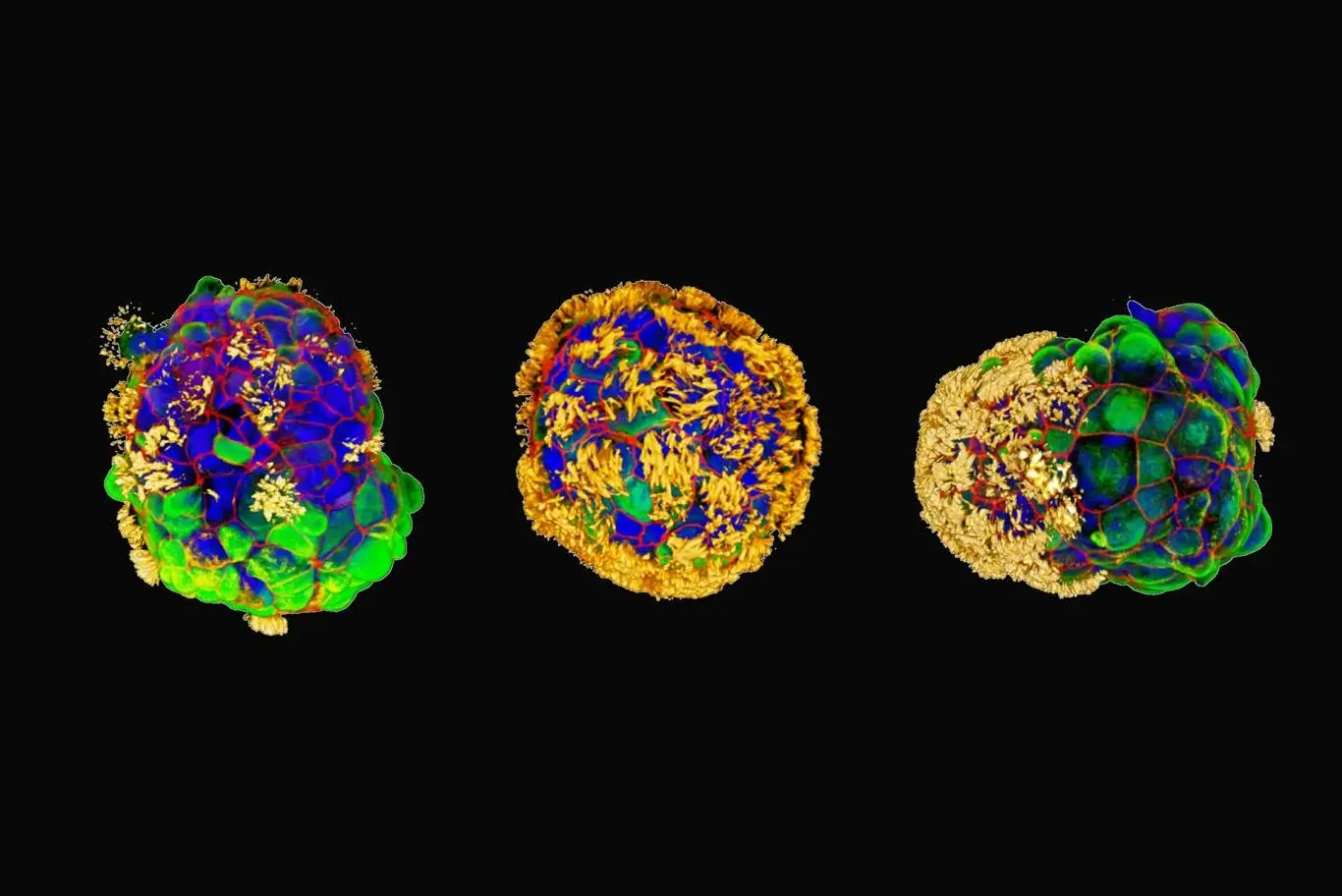
Each anthropobot started life as a single cell, coming from an adult human donor. The cells are collected from the surface of the trachea, and are covered with fine hairs called cilia that wave back and forth. The original function of the hairs in the trachea is to push out tiny particles that find their way to the airways and lungs. When the cells were grown in the laboratory, they spontaneously joined together into a tiny sphere made up of many cells.
The researchers found certain growth conditions that caused cilia - hairs - to grow on the surface of the balls. The hairs whip around them like tiny paddles, and move the balls in the solution. This, of course, is in complete contrast to their original role in the body. They were never intended to be used for propulsion! But it doesn't matter to anthropoids. The cilia do the job well enough that they can move around the petri dish. As the famous verse says - "nature finds solutions". What works, works.
As of now, it seems that the balls are developing in several different ways. Some are spherical and covered with cilia all around. These, logically, do not advance too much. The hairs push in all directions to an equal extent, so the spherical anthrobots are mostly content with side-to-side vibrations. Other anthropoids come in an elliptical and less symmetrical shape, with cilia that only grow in certain areas. These tend to move in more interesting ways: in straight lines, circles and arcs. All types survived for a maximum of sixty days, before disintegrating and decaying.
Before we continue, it is important to clarify what the anthrobots are gone can do right now. They cannot sense their surroundings accurately. They can't even perform complex actions like the xenobots. But it's not surprising. This is the first time that such anthropoids have been built from human cells, and if there is anything we have learned from the original xenobots, it is that if we add more types of cells to the anthropoids in the right ways - they will also acquire new abilities.
So yes, I admit that the anthropoids don't sound too impressive right now, but the principle is what matters. That is why I will return for a moment to the magnitude of the act: adult human cells, which had been sitting for decades in the trachea of an unknown person, were given by the researchers an opportunity to withdraw from the body, reproduce and reconnect in unique and creative ways. Perhaps it is not surprising that Gizm Gomusakya, the main researcher, is actually an architect by origin, especially when you hear her talking about the new robots -
"By reprogramming the interactions between the cells, it is possible to create new multicellular structures, similar to the way stone and bricks can be organized into different structural elements such as walls, arches and columns."
In short, it is a new type of Lego: biological Lego, in which the cells make up the different stones. In this study only one type of Lego bricks was used, and the results accordingly. In the following studies and developments - the sky will be the limit.

The little healers
One hope is to eventually use the anthropoids to heal the body from the inside. Because of this, the researchers created a special environment to test whether the robots could help wounds heal. They grew a layer of nerve cells on a Petri dish, until their extensions filled the entire dish and intertwined and hugged each other, like the top of a large forest. Or, in other words, like dense tissue.
Then came the time of destruction. The researchers scraped the surface with a metal stick to injure the cells and leave an exposed trail in the tissue. After creating this 'wound', they injected the anthrobots directly into that open area between the nerve cells and let them sit there and do... nothing. At least as far as we know. They didn't even make too much progress. As I said - these are still not the most advanced robots in the world.
We would expect that the anthrobots would not have a positive or negative effect on the way the nerve cells grow. After all, the original role of these cells in the body is not to heal tissue. But surprisingly, the anthrobots caused the nerve cells to bridge the wound quickly. The nerve cells were attracted to the anthropoids, multiplied and filled the damaged area.
What happened in the areas where the anthropoids were not present? The nerve cells did not agree to go there either. The wound remains open and gaping in the tissue.
These are exciting results...carefully. They imply that we could use the distant-far-far-distant descendants of the anthrobots, the kind that will be developed in the coming decades, to bridge spinal cord injuries. Perhaps the anthropots of the future will give a chance for a better life to people who suffer from paralysis due to spinal cord injury. As of today, this is only an experiment in the petri dish. But the potential is already clear today.
"The cellular structures we assembled in the laboratory exhibit abilities that surpass those they possess in the body," said the study's director, Professor Michael Levine of Tufts University and Harvard. "It is fascinating and completely unexpected that normal cells from a patient's trachea, whose DNA has not been re-edited, can move by themselves and encourage the growth of neurons in damaged areas. We are now examining how the healing mechanism works, and asking what else these robots can do."
Levin also understands that one study in a Petri dish (as interesting as it may be) certainly does not mean that the anthropoids will have a similar effect inside the body as well. But we already know that there are cells that circulate in the body and help us heal wounds and fight infections: the blood platelets, for example, or the white blood cells. There's no reason to think that the anthropots won't be able to achieve similar feats sooner or later, as we advance in biological lego assembly skills.
The promising future of the anthropots
What could a world look like in which anthropoids realize their full potential?
In such a world I could donate my body cells in tiny amounts: no more than a throat swab, or a blood sample of a few milliliters is needed. Those cells will be carefully and lovingly grown in the laboratory by robo-researchers, and reassembled into forms that can perform specific tasks in the body. The whole growing process will take a few weeks in total, and at the end of it I can go to the doctor and get a heaping dose of anthropotes by injection.
What will the anthropots do in my body? It already depends on the way they will be engineered. Some will be engineered so that they can circulate in the central arteries and clean them thoroughly to prevent heart attacks. Others will transport calcium to the bones of the elderly to prevent osteoporosis. A third type will go around the body tissues themselves and look for cancer cells, or they will focus directly on tumors that they can kill. And if, God forbid, I injured my back and became paralyzed, then dedicated anthropoids would be able to migrate and settle in the space between the nerve cell extensions, and encourage them to reconnect. After they finish their job, the anthrobots will break down and be absorbed into the body without causing any damage.
And these are just a few of the most obvious abilities - the ones that it goes without saying that we will covet - that the athenrobots may boast of in that future.
But what other abilities might the anthropoids develop?
The less promising future of the anthropoids
When we think about the great opportunities that the future opens up for us, it is important to shed light on the risks as well. Yes, the anthropots will one day be able to heal the body, but what else will they be able to do with it?
First of all, we need to clarify everything we don't know yet - and that's a lot. We do not know, for example, whether the cells that make up the robots may not develop harmful mutations, especially when injected into the body. Why would such mutations develop? A better answer is - why not. Even the most mature human cells may, in rare cases, develop mutations and get out of control. This is all the more true for cells that are cut off from their normal place of growth and are forced to reconnect with other cells in different ways. Shocks of this kind can cause cells to respond in ways we are not yet aware of. This is a risk that we will have to understand and prepare for through laboratory and clinical trials.
Even if the anthropoids do exactly as they are supposed to and don't get out of control, they could make an excellent biological weapon, especially if we manage to give them the ability to reproduce on their own. This is not science fiction: the original frog xenobots showed that they could indeed replicate. They collected frog cells from the solution in which they were grown, and recombined them to make copies of themselves over several generations. Granted, they only did so because they were carefully designed to do so, so there is no immediate reason to think that the simple anthropots would be able to reproduce. But in the future, as mentioned, they will not be simple. And if they are shot to reproduce - they will probably be able to do so.
Now, how does an army of robots sound to you, the size of each of which does not exceed that of a pinhead, and they are able to invade the body, reproduce and successfully fight all its defense systems? It is already a less promising future. But it is also opening up to us these days.
I would like to say that I hope the human race does not use its advanced engineering skills to develop new weapons. But this is the human race, so of course we will do that too. And at the same time, it is clear that we will also develop advanced defense measures against these biological weapons.
Let's hope, at least, that this type of warfare will never be widely used. Let's hope that the human race will know how to use the anthropoids of the future more to heal, and less to kill. A future where our body cells will be used to help us all - and not start revolutions against us.
Source: Tufts University press release
More of the topic in Hayadan:
