The cell's garbage disposal system changes in cancer and allows it to escape the immune system
You opened Facebook and saw that you had a new friend request. The friend requester's name is familiar to you, but he has few friends and plenty of strange advertisements on the page. Will you approve the request? Similar to the judgment that helps us identify imposters on social networks, our immune system knows how to identify dangerous cells based on the cellular "profiles" they present. The cells of the immune system cruise the body and examine proteins called "antigens" displayed on the cells. When the antigenic profile of a certain cell raises suspicion, the system works to destroy it.
The antigens that make up the cellular profile are produced, among other things, by a system called the proteasome - a kind of cellular garbage can that specializes in breaking down proteins that have finished their function or have been damaged over time. These proteins are put into the proteasome where they are broken down into peptides - shorter protein chains - some of which are intended for recycling, and some of which are sent for further processing which will eventually be displayed on the cell as antigens. Cancer cells often present suspicious antigens, since various control systems of the cell go wrong in cancer and increase the chance of abnormal production of proteins. When the immune system is exposed to the suspected cancer profile, it can attack, but in practice, for various reasons, this does not always happen. In a new study thatRecently published, researchers from the laboratory of Prof. Yifat Marble In the Department of Systemic Immunology at the Weizmann Institute of Science, they buried their hands in the depths of cellular garbage and pulled out a mechanism by which cancer cells disrupt the ability of the immune system to recognize them.
This is not the first time that Prof. Marble's laboratory has put the cellular waste removal system under the microscope. In recent years the members of the laboratory developed advanced technologies which allow them to map the proteins that undergo degradation in the proteasome. The information is obtained in high resolution, so that the unique structure of a peptide that has been cut by the proteasome can be identified.
Imagine that the different proteasomes are two chefs who prefer different seasonings. The basal proteasome likes to cook sour peptides, while the immunoproteasome makes sweet peptides that attract the cells of the immune system
In the new study, led by Aharon Javit and Merev Shmueli, these technologies were used to examine for the first time the cellular garbage cans in cancer. "We took this system and tried to understand what happens to the removal of the garbage and the proteins broken down in it in cancerous tissues of patients with the most common lung cancer. When we made a comparison between the cutting products of the proteasome in the cancer tissue and those from a nearby non-cancerous tissue, we saw that the same proteins undergo a different type of cutting", says Prof. Marble. The findings raised the idea that perhaps we need to look at the proteasome from a new angle - to check how the protein breakdown factory works, and not just what products passed through it. "We looked at the cancerous tissue and asked - how does the proteasome change?"
A proteasome is a complex of proteins made up of subunits: the center of the proteasome is a kind of hollow barrel that contains various enzymes that specialize in cutting the cellular garbage, to which other proteins can be attached that serve as a kind of caps that regulate the entry of proteins. Proteasomes differ from each other mainly according to the enzymes that make up their center. For example, the composition of the enzymes of a proteasome known as the "constitutive proteasome" is responsible for certain types of cuts, while a proteasome with other components, known as the immunoproteasome, specializes in cutting peptides that are particularly suited to be used as antigens - those cellular profiles that can help the immune system identify Undesirable factors.
When the researchers went to rummage through the garbage cans of lung cancer tumors removed from patients, they noticed a significant increase in the number of proteasomes with the protein PSME4, compared to the level of expression of the same protein in the proteasomes of adjacent non-cancerous control tissues. The PSME4 protein is one of the various caps that can assemble the proteasome, but it is evident that its use is not particularly common in non-cancerous tissue.
The Proteasome Cookbook
After the researchers saw that the PSME4 protein did bind to proteasomes in the cancer samples, they began to characterize the nature of the cleavage of the proteasomes in which it is expressed. To illustrate, imagine that the different proteasomes are two chefs who prefer different seasonings. The basal proteasome likes to cook sour peptides, while the immunoproteasome makes sweet peptides that attract the cells of the immune system. Using complex biochemical methods, the researchers showed that as the concentration of the PSME4 protein increases, the cell produces more of the acidic portions, while the amount of sweet peptides decreases. In a series of follow-up experiments, the researchers showed that the imbalance between the types of sour and sweet antigens makes it difficult for the immune system to characterize the cellular profile, thus preventing a desired immune response that should fight the cancer cells.
These findings led to the hypothesis that the level of expression of the PSME4 protein may influence the response of cancer patients to immunotherapy - the new generation of cancer treatments that recruits and activates the immune system against the malignant tumors. Therefore, the researchers turned to collaborative databases that include data on different cancer tumors and the reactions of patients to different treatment methods. They discovered that the composition of the proteasome varies beyond recognition between one type of cancer and another, and each cancer has a different garbage disposal system. In addition, they saw that the increase in PSME4 protein expression did not characterize all types of cancer, but overall it was evident that the higher the level of the protein was in the cancer, the lower the patients' response to immunotherapy treatments.
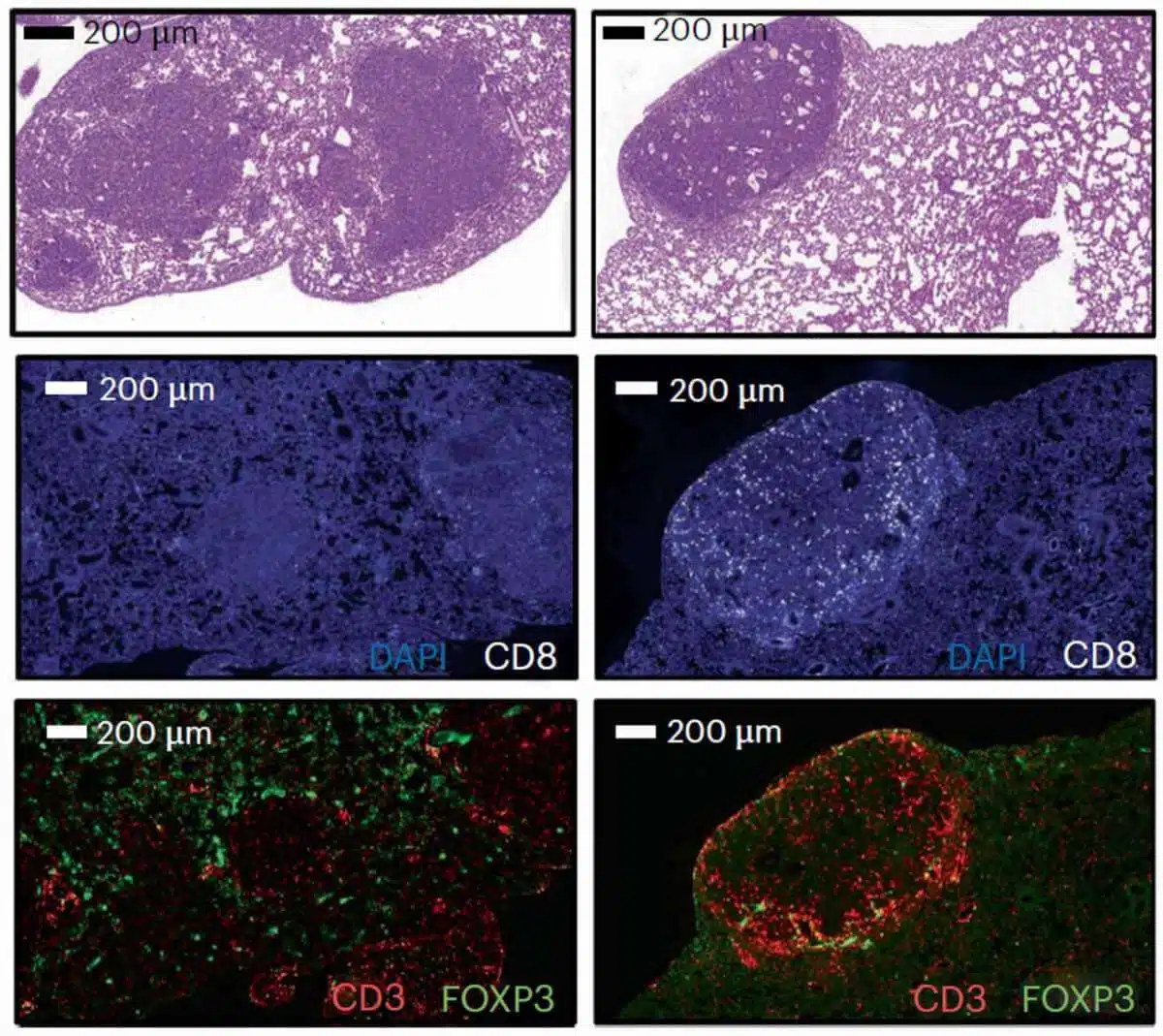
To verify that the PSME4 protein affects the activity of the immune system directly, the researchers conducted a series of experiments in a model of lung cancer in mice. These tests showed that the expression level of the PSME4 protein is so significant for cancer survival that mice engineered to express more of the protein in the cancer cells developed tissues full of cancerous tumors. In contrast, the researchers barely detected cancerous tumors in mice engineered to express little PSME4 in the cancer cells. Finally, when they looked at mice that do not have an immune system, they saw that the tumors were not affected at all by the level of expression of the protein, a finding that strengthened the hypothesis that PSME4 affects cancer through the immune system.
"Our research focused on non-small cell lung cancer (NSCLC), but the data we published indicate that there are other types of cancer in which the PSME4 protein is expressed at a high level," concludes Prof. Marble, and emphasizes the need to look into the insides of the cancer's garbage disposal system, where There are hidden additional mechanisms that influence the activity of the immune system. Now the researchers aim to develop a drug that can reduce the expression levels of the PSME4 protein in the cancerous tissue, thus possibly significantly improving the ability of the immune system to attack the cancer. Although the road to drug development is still long, the findings demonstrate how the characterization of the proteasome in the cancer tumor can help doctors make a personalized decision regarding the right treatment for the patient.
