An algorithm developed by the institute's scientists heralds a leap forward in the field of humanizing antibodies and may enable the rapid, efficient and cheaper development of new drugs. The key: stability
In the late 19th century, doctors began pioneering attempts to vaccinate and cure humans using antibodies derived from serum extracted from horses. Most of these attempts led to devastating results - the encounter of the human body with the foreign antibodies produced an acute and even fatal immune response. Only decades later, towards the end of the 20th century, scientists were able to adapt animal antibodies to the human immune system through a process known as "humanization" - that is, replacing parts of the animal's antibody with human segments so that it would not be recognized as an invader. Although there have been achievements on this front, the creation of these hybrid antibodies is an arduous, expensive and long process, and in the end the antibodies obtained may be less effective or even completely useless. In a study published in the scientific journal Nature Biomedical Engineering Weizmann Institute of Science scientists presented an algorithm for designing humanized antibodies that operates on the basis of a new principle that is expected to significantly shorten and reduce the process and even revolutionize the field of antibody-based drug development.
Antibodies are proteins that are formed in our body in response to various pathogens. Like a key to a lock, each of them has a unique structure capable of neutralizing a specific target thanks to a high spatial and chemical compatibility with that target. The antibodies are built in the shape of the letter Y, and while the "stem" of the Y does not change that much between one antibody and another, the wide open "hands", and especially their tips or "fingers", are built in each antibody in a unique way that allows it to recognize the target and bind to it . Antibodies from animals often have "fingers" that can bind to a human pathogen, but the rest of them may be recognized by the immune system as a foreign invader that must be defeated.
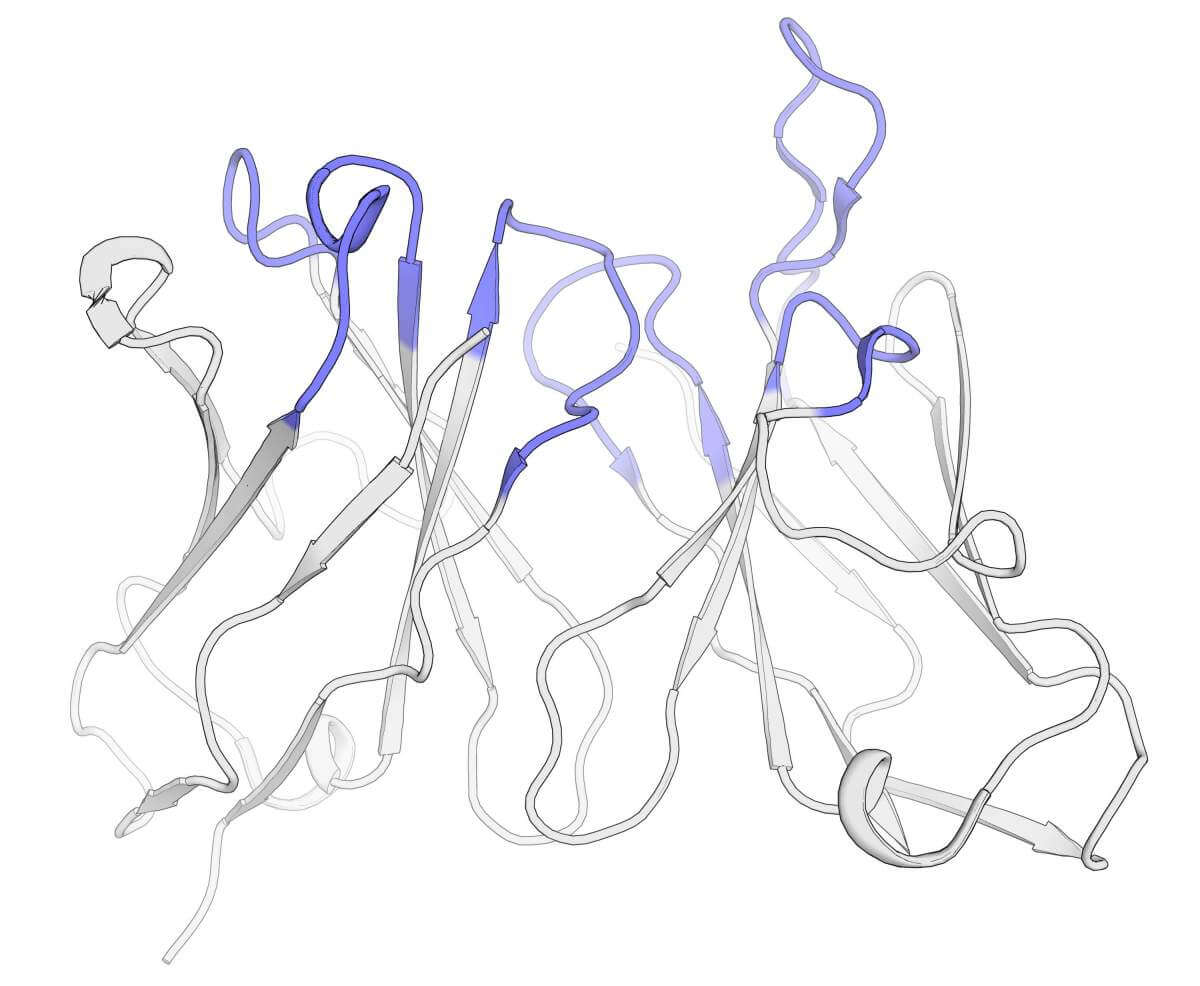
"The work of humanization is complex and complicated. On the one hand, parts of the antibody must be replaced with segments that the human body knows in order to prevent a life-threatening immune reaction, and on the other hand, any change in the original antibody, especially in its fingertips, may undermine its stability or impair its ability to recognize the target," says Prof. Sheral Fleishman from the department of biomolecular sciences at the institute whose laboratory is a sort of development center for algorithms for protein design and planning.
In the last forty years, the way used to develop drugs based on animal antibodies relies on the principle of similarity. That is, the researchers look for the human antibody most similar to that of the animal, and inject parts of it into the animal antibody - while keeping the parts that are supposed to act against the disease-causing agent. But even if you manage to avoid changes in the fingertips in this process, because all the parts of the antibody are connected and dependent on each other - any change may destabilize the entire protein or simply make it ineffective. "It's like moving the interior walls of a house without taking into account the foundations of the building," explains research student Ariel Tanenhaus.
Knowing that the known methods for humanizing antibodies often result in the loss of activity and stability of the antibody, the researchers took it upon themselves to rethink the guiding principles of the process. Tanenhaus, who led the research in Prof. Fleishman's laboratory, wanted to test what would happen if instead of choosing human segments based on similarity to the segments of the original antibody, they chose the segments based on the stability they would impart to the final structure. In other words, he decided to prefer "stability" over "imagination". To test the effectiveness of the strategy, Tanenhaus developed an algorithm called CUMAb, whose job it is to suggest potential designs for humanizing antibodies based on existing databases of human antibody segments, and to rate the structural stability of each of the designs.
In collaboration with several research groups at the Weizmann Institute and the University of Michigan, the scientists examined which designs the algorithm would suggest for each of the five murine antibodies selected to participate in the study. One of the antibodies chosen was an anti-cancer protein from the laboratory of Prof. Deborah Fass in the department of structural and chemical biology at the institute. Previous attempts to create humanized versions of this protein to develop a drug from it have failed. The algorithm suggested an unprecedented amount of about 20,000 different humanization options for this antibody, and even rated the level of stability of each design. The researchers selected the highest rated proposals, created the humanized antibodies in the laboratory and tested their function.
"The results were spectacular," says Prof. Fleishman. "The humanized antibodies will function with an efficiency similar to that of the original mouse antibody, and this without us having to make any adjustments after the calculation." Equally impressive were the results obtained for the other four antibodies: each of the humanized antibodies performed identically to the mouse antibody, and in some cases the humanized proteins were more stable or worked better than the original antibodies.
In light of the great potential inherent in these findings and the possibility of revolutionizing the field of antibody humanization, the researchers decided to make the algorithm available to the scientific community and it is currently available to the research public online. "This is the first method that shows such promising results in the field of antibody engineering. We hope that the algorithm will change the way in which antibody-based drugs are developed and will make it possible to speed up the pace of their release to the market in order to improve the condition of patients", says Prof. Fleishman.
Beyond the issues of humanization, the new algorithm can solve other problems related to antibody engineering. For example, antibody-based drugs may be too viscous to use by injection into patients. Using CUMAb, the researchers hypothesize that it will be possible to engineer antibodies that are less viscous or have other properties that will improve the quality of treatment.
Lev Khmelnitsky, Noa Yeshaya and Prof. Deborah Fass from the Department of Structural and Chemical Biology at the Institute also participated in the study; Razi Khalaila, Dr. Ashish Nuruna, Dr. Mosheit Lindzen, Prof. Yaacov Abramson and Prof. Yosef Yordan from the Department of Immunology and Biological Regeneration at the Institute; Dr. Emily K. McCawsky and Prof. Peter M. Tessier of the University of Michigan; Dr. Ira Zaretsky, Dr. Yael Friedman Sirkis and Yael Galon-Wolfenzon from the Department of Life Sciences Research Infrastructures.
More of the topic in Hayadan:
