The test is based on a technology developed by the group led by Prof. Amit Meller based on individual molecules without the need to duplicate many copies of the same molecule
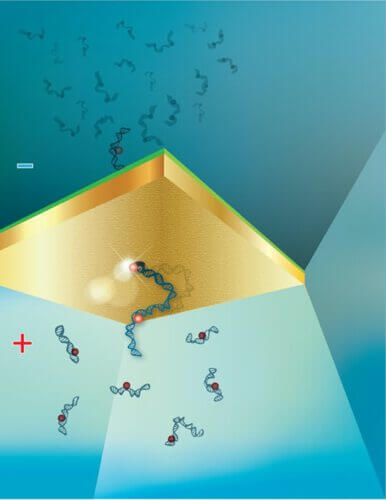
Researchers at the Technion have developed a highly accurate method for diagnosing corona without the need for PCR amplification. With this method, the presence of the SARS-CoV-2 virus in a sample is detected based on the counting and quantification of individual RNA molecules.
the research published in the journal DHW Nano It was led by Prof. Amit Meller and the post-doctoral student Dr. Yana Rozevski and participated in by Dr. Tal Gilboa, Dr. Xander Van Koten and Dr. Diana Hutner - all researchers in the Faculty of Biomedical Engineering at the Technion - and Prof. Ulrika Stein from the Max Delbrück Center for Medicine Molecular and the Sarita Hospital in Berlin.
The RT-qPCR test, which is the test currently used in the diagnosis of Covid-19, is based on a series of preparation steps that include collecting the sample from the subject using a swab, "opening" the virus to reveal the genetic material inside it and extracting the genetic material of the RNA. Then comes the RT stage, and in Hebrew "reverse transcription", where the RNA sequences are translated into DNA sequences, followed by the PCR stage ("exponential amplification") which repeatedly doubles the DNA molecules To reach a quantity that allows sampling them in solution and determining if it is indeed SARS-CoV-2 DNA.
RT-qPCR testing is a lengthy process that requires special materials (reagents), expensive laboratory equipment and experienced professionals. Furthermore, recent studies indicate that the test results may vary from day to day and that significant errors may occur in the massive amplification process. This is the background for the worldwide effort to develop faster, cheaper and more accurate methods. This is a very complex challenge in itself, and it is especially complex when the virus molecules are few and do not occupy a significant part of the sample.
The diagnostic method presented by Prof. Meller's research group in the article is based on an original technology that he developed in the last decade, a technology whose effectiveness has already been demonstrated in many other contexts. Its purpose: single-molecule sensing, that is, clinical diagnosis based on the analysis of single biological molecules, without the need for large samples containing many copies of the same molecule. This technology, developed in part for the purpose of diagnosing cancer cells based on biological markers, is based on pulling biological molecules such as DNA, using an electric field, into a nanometer hole containing electrical or optical sensors. The electronic output goes through a computational analysis that allows direct and immediate identification and counting of the molecules. This approach opens up the possibility of miniaturizing molecular sensing systems while improving the accuracy and reliability of the tests and extending them to cases where PCR amplification is ineffective or impairs the reliability of the test.
The current article presents applications of this method in two contexts: the detection of RNA molecules that report the formation of metastatic cancer and the diagnosis of corona. In both cases, the researchers developed a process for enzymatic degradation of all background molecules except the relevant target molecules. In the first context, the researchers demonstrated the potential of the method for the early detection of metastatic cancer, by quantifying the intensity of expression of MACC1 - one of the genes essential for the transition to a metastatic state. Thanks to its great sensitivity, the new technique was able to accurately quantify the expression of the gene in cancer cells from the beginning of the disease - a challenge that PCR-based technologies failed at. It goes without saying that as the genetic markers of cancer are discovered at an earlier stage, the chances of treatment success increase greatly.
In the second context, the researchers quantified the RNA molecules of the SARS-CoV-2 virus using the same method. The method presented in the article, RT-qNP, is not the first method for the analysis of a single molecule, but unlike its predecessors, it eliminates preliminary processes that introduce "noise" and inaccuracy into the system. Two of these "noisy" processes are the purification of the sample, which leads to the loss of most of the significant biological markers, and the amplification of the DNA molecules, which as mentioned leads to errors and misdiagnosis.
According to Prof. Meller, "Our method allows for quantitative sensing of the genetic expression of the molecule using a relatively simple nanosensor-based device, without the need to purify the sample and without the need for amplification - processes that harm the sensitivity and reliability of the test. As we have shown, our technology preserves throughout the process the level of genetic expression of the original RNA molecules. This is how a more accurate analysis is achieved, which is essential in the two contexts under discussion - metastatic cancer and the SARS-CoV-2 virus."
For Prof. Meller's research group, the current article is a very important landmark, but it is not the end of the story. The sensing system based on nanonozzles will become a portable device and eliminate the use of cumbersome laboratory equipment. The research continues into technological and clinical channels at the Faculty of Biomedical Engineering at the Technion while collaborating with the sample bank (biobank) at the Rambam Medical College. At the same time, steps are being taken to commercialize the technology in order to bring it to wide use as soon as possible.
The research was supported by the European Union (ERC grant under the Horizon 2020 program of the European Commission for Research in the European Union), the National Science Foundation (ISF) and the SignGene program that supports PhD students.
for a magazine article ACS NANO
More of the topic in Hayadan:
