Azobenzenes are versatile compounds with many potential uses, such as advancing technology through the production of tiny machines, as well as creating light-activated drugs. These molecules can be found in two different forms called "E" and "Z" which can be exchanged between them through irradiation. However, under irradiation conditions both forms are in equilibrium, which prevents optimal utilization for different applications
A new discovery allows scientists to change the shape of azobenzene molecules using visible light - an easier and safer way than the ultraviolet light used in the past. Azobenzenes are versatile compounds with many potential uses, such as advancing technology through the production of tiny machines, as well as creating light-activated drugs. These molecules can be found in two different forms called "E" and "Z" which can be exchanged between them through irradiation. However, under irradiation conditions both forms are in equilibrium, which prevents optimal utilization for different applications. The ability to control this process using visible light and prefer only one form over the other opens up new possibilities for these applications that become more accessible and efficient.
Azobenzenes are versatile molecules with enormous potential in many technological applications, which traditionally required the use of energetic ultraviolet radiation in order to activate them. However, a new approach promises to change the rules of the game.
The team of researchers led by the scientists Prof. Igor Shapiro from the Hebrew University of Jerusalem, Raphael Klein from the Weizmann Institute of Science and the Austrian Institute of Science and Technology together with Ari Primagi from the University of Tampere in Finland, introduced a new concept called "de-equilibrium by sensitization in a trapped state" or (DESC) in English. This innovative approach offers for the first time the possibility of causing the molecular shape change using visible light, including wavelengths in the red part of the spectrum.
The study, titled "De-equilibrium by increasing sensitivity to visible light under confinement conditions", was recently published in the prestigious scientific journal, Science.
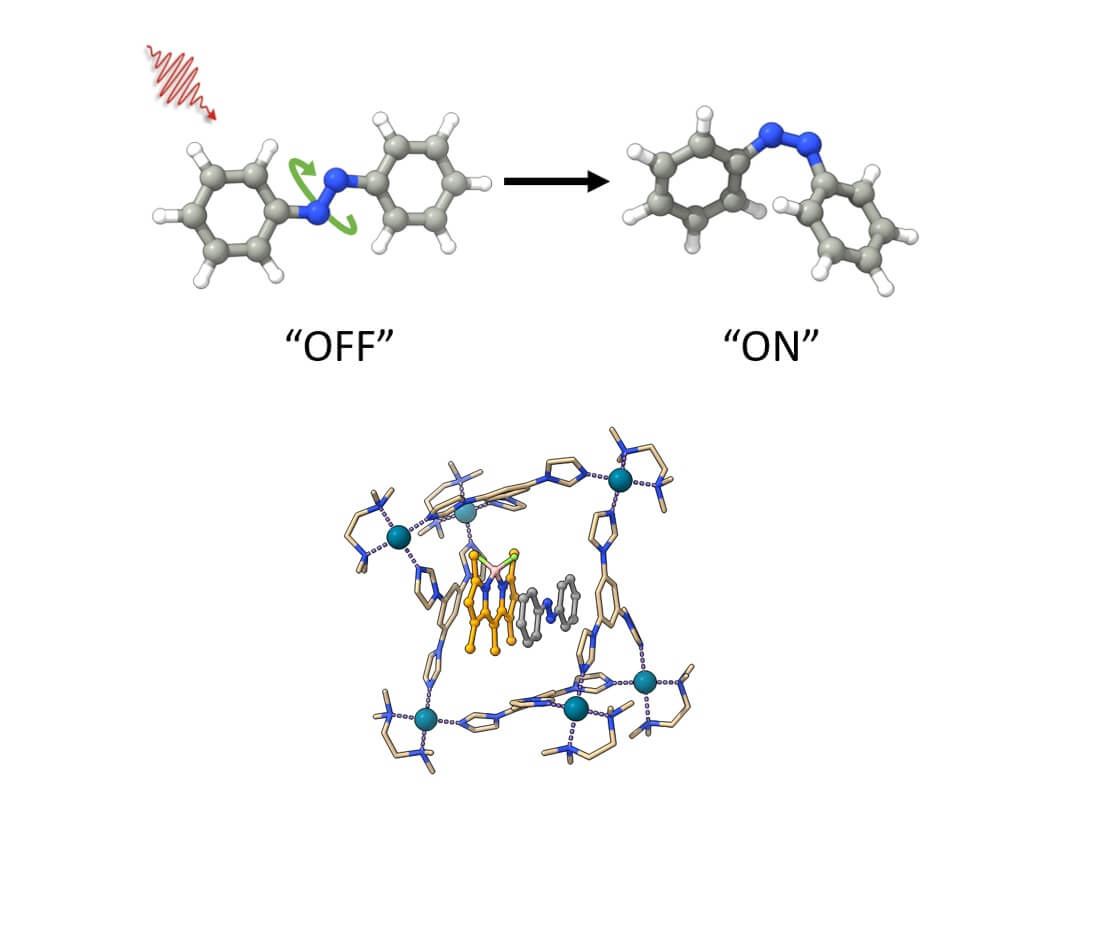
What makes azobenzene molecules particularly interesting is their ability to undergo changes in shape in response to irradiation at specific wavelengths, such as ultraviolet light or visible light. This phenomenon, known as photoisomerization, allows azobenzenes to switch between two separate forms or isomers, called "Z" and "E", which can be thought of as an "on" state and an "off" state, respectively. This unique property is of enormous significance, as it opens up a wide range of applications including nanotechnology, data storage, drug delivery, materials science, and biological research.
"Through computer simulation of the isomerization process using quantum chemistry, we clarified the mechanism behind this innovative approach that not only advances basic research in the field of azobenzenes, but also paves the way for many practical uses. These applications harness the power of visible light, and in particular red light, which has the lowest energy in the spectrum, and prevent the use of ultraviolet radiation, which is dangerous and carcinogenic," says Prof. Igor Shapiro, from the Hebrew University of Jerusalem.
The main points of the study:
New approach: DESC presents a novel supramolecular approach to the photoisomerization of azobenzene, enabling it with visible light, including red wavelengths.
Teamwork of molecules: the combination of a special macrocyclic host and a photosensitizer is the secret behind the success of DESC. They work together to identify and modify certain azobenzene molecules.
Opening up new opportunities: DESC leading to special photostationary states that could not be created directly with light before. This opens up many new possibilities for using azobenzenes in different ways.
"We were able to capture a molecular film using time-lapse spectroscopy, which sheds light on the complex operation of our discovery. This cinematic insight was used to verify the underlying mechanism of imbalance by sensitization under confinement conditions (DESC), and provides empirical evidence for the ground-breaking potential of this groundbreaking supramolecular approach". said Prof. Ari Primagi, from the University of Tampere
This pioneering research opens new and exciting ways to use azobenzenes in various fields. By expanding the range of wavelengths that can cause isomerization, DESC promises to improve the efficiency and application of azobenzene-based technologies.
More of the topic in Hayadan:

2 תגובות
The article was written by the Technion spokeswoman.
No one does pronunciation before uploading an article? And what else do they know?!
Some errors and repetitions and chewing material. Too bad, actually a fascinating object