Before the successful synthesis, it was not clear if it was even possible to create such a molecule and keep it stable long enough to study its structure and its electronic properties.
[Translation by Dr. Moshe Nachmani]
Chemists from Great Britain and Switzerland succeeded in synthesizing the carbon ring prod 16C and showed the first evidence of its antiaromatic character. The rings from this family constitute a new configuration of the element carbon, and therefore they are defined as a new allotrope of it
In 2019, researchers from the University of Oxford and the IBM Research Institute in Europe (Zurich) reported the synthesis of a new allotrope of carbon, 18C, a cyclic polyene consisting of eighteen carbon atoms connected by alternating single and triple bonds. The molecule is also doubly aromatic, thanks to the two pi systems perpendicular to each other - one in the plane of the ring and one out of this plane and perpendicular to it.
![The starting material 4 is deposited on a surface of sodium chloride; The carbon monoxide and halogen chemical groups are then removed to obtain the 16C carbon ring [Courtesy: Yueze Gao et al 2023]](https://www.hayadan.org.il/images/content3/2023/10/529837_full_text__anderson_41586_2023_6566_onlinepdf_3002_599431.jpg)
Now, two research groups, one from the University of Oxford (Harry Anderson) and the other from Zurich (Leo Gross), have taken the idea of carbon rings one step further by creating a new candidate: a ring with 16 carbon atoms. Unlike the 18-carbon ring which is aromatic, the 16-carbon ring is expected to be doubly antiaromatic and therefore unstable. Before the successful synthesis, it was not clear if it was even possible to create such a molecule and keep it stable long enough to study its structure and its electronic properties.
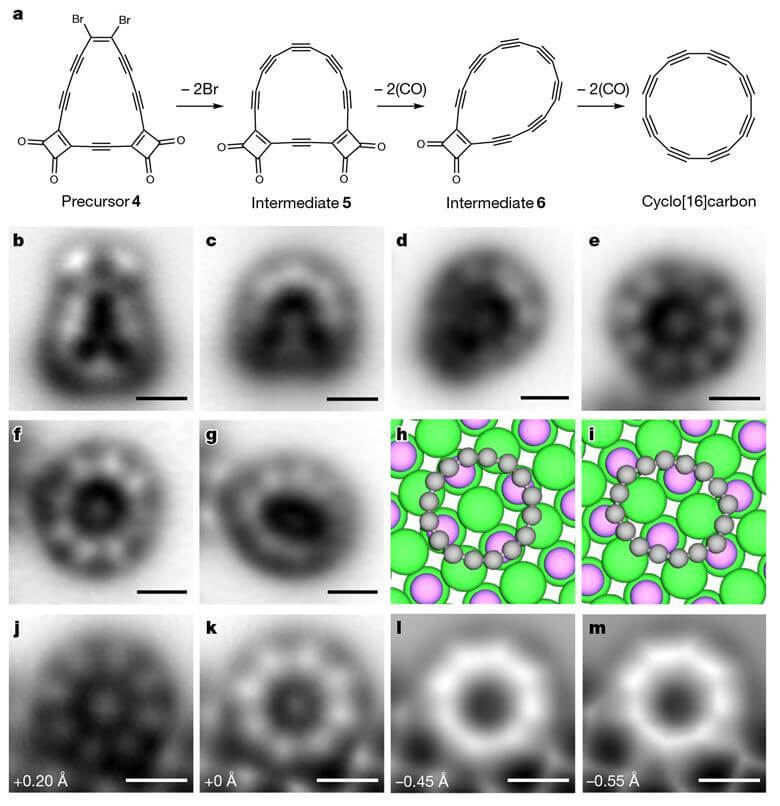
the starting material 4 deposited on a surface of sodium chloride; The carbon monoxide and halogen chemical groups are then removed to obtain the carbon ring 16C [Courtesy: Yueze Gao et al 2023]
"The challenge in the synthesis of ring 16 was mainly the instability of the starting materials, which were also antiaromatic," explains the lead researcher. The macrocyclic starting materials contained several chemical groups that contribute to the stability of the molecule. These groups can be removed at a later stage and become alkyne bonds (triple bond) by removing the masking groups. Initial attempts to use masking groups similar to those used in the synthesis of ring 18 proved too unstable for the required material. The ultimately successful approach involved a macroring with four groups of carbon monoxide and two groups of bromine atoms. In the next step, this material is deposited on a sodium chloride surface. In the next step, the researchers used an electric current to remove all these groups and obtain the required final product.
Using an atomic force microscope confirmed the structure of the ring consisting of alternating single and triple bonds. The researchers were also able to analyze the distribution of electrons within the molecular orbitals of the neutral molecule, which includes 16 carbon atoms, as well as that of its anions with the help of an atomic force microscope and a scanning tunneling microscope. These measurements together with theoretical calculations verified the hypothesis that the electronic ground state of the molecule is indeed doubly antiaromatic.
In light of all these findings, the molecule provides new opportunities for experimental research of the theories involved in such structures. "Organic chemists like to discuss and debate the idea of aromaticity, and the unusual synthesis of a 16-double antiaromatic ring will certainly generate controversy and new insights," explains the lead researcher from the University of Alberta, Canada.
The cyclocarbon family is expected to grow even more soon. The research group in China has already written three new papers that are currently under peer review regarding the syntheses of carbon rings consisting of only 6, 10, 12, 14 and 20 carbon atoms. These articles cover the full range of carbon rings that are now believed to be stable enough for research. With a wide spectrum of new molecules to research about, the next step will be to examine their reaction with each other, to create yet another host of new molecules. "The next breakthrough in this field could be the discovery of methods to control the reaction of cyclocarbon molecules with each other to create extensive allotropes of carbon," says the lead researcher.
The news about the discovery (on the website of the Royal Society of Chemistry)
More of the topic in Hayadan:

One response
So many mistakes in one article... Come on.