It turns out that the shorter a crack in brittle materials - the more resistant they are to it, which can help in the design and use of applications that are based on brittle materials

How resistant are materials to cracks and can they be prevented from breaking? Brittle materials have low fracture energy, meaning they break easily. These materials are used, among other things, in the microelectronics industry, energy, biology and for many applications such as substrates for printed circuits (the boards on which electronic components that form an electric circuit are placed, for example in computers and telephones), etc. in batteries, optical components and devices inspired by nature (Bio-Inspired Materials). That is why it is important to know their properties and know how to prevent cracks from spreading inside them, in processes that may lead to their breakage.
What is the question? What does the material's resistance to the progression of cracks in it depend on?
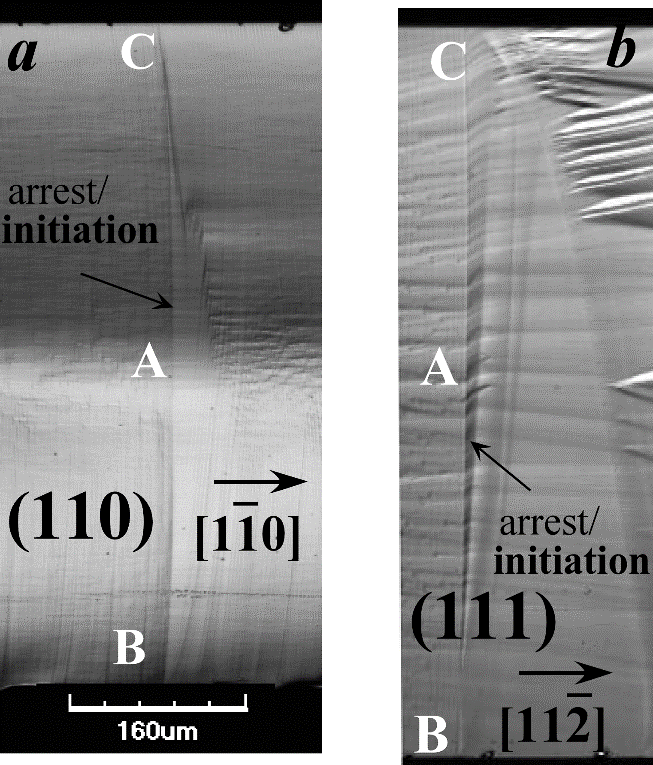
Prof. Dov Sherman from the School of Mechanical Engineering at Tel Aviv University studies mechanical properties of materials. In doing so, he focuses on the progression of cracks in brittle materials and especially in single crystals - in which the atoms are arranged in a regular periodic manner (such as silicon, quartz and lithium niobate) and are considered more fragile. At the same time, he examines the theory of the fracture developed 100 years ago by the British engineer Alan Arnold Griffith (AA Griffith, 1921). This theory makes it possible to formulate the strength of the material in the presence of cracks. According to which, in every material there are cracks of varying sizes and directions, and when force is applied to it, the large cracks spread rapidly and cause it to break. In addition, she holds that the property of the material that resists the initiation and progression of a crack is the free surface energy of the material because a crack creates two new free surfaces. This theory contributed to the design of materials despite the cracks in them. A photograph of the fracture surface in a silicone model, across both fracture planes, shows that the crack profile has a certain curvature
"Griffith established the laws according to which cracks progress in materials, and I examine them again through experiments and calculations. As mentioned, every material has a feature of resistance to crack growth, but in brittle materials it is relatively weak. I check with greater precision which properties in materials cause them to break and how they can still be designed, so that there are as few cracks as possible and that they are as short as possible," says Prof. Sherman.
As part of their research, Prof. Sherman and the students in his group cut models from industrial silicon wafers and create thin grooves in them using a laser beam. Then they heat them on an electric plate at about 200 degrees and then dip them in a saucer of water. This creates a thermal shock (heating and then rapid cooling) that causes natural cracks (the thickness of an atom) of varying lengths, according to the height of the water. Then they glue the cracked models using epoxy glue inside a rectangular opening in an aluminum plate. After the glue hardens, they put them on an electric hotplate and heat them at five to ten degrees. Since aluminum expands much more than silicon, the silicon stretches and the cracks progress quickly. At this stage, Prof. Sherman calculates, using dedicated software, the energy level for the fracture of the material (the level of its resistance to the progress of cracks within it). In addition, he measures the speed of the cracks' progress, according to the electrical voltage across the models, with a dedicated measuring device (a fast oscilloscope - reflects fluctuations).
The scientists discovered that the rate of resistance of the material to the initiation of cracks depends on their length. The shorter the cracks, the greater the material's resistance to them. And the longer the cracks - the weaker the material's resistance to them. The difference in the fracture energy between the two states is very significant, up to three-four times.
Thus, among other things, Prof. Sherman and his team discovered that the rate of resistance of the material to the initiation of cracks depends on their length. The shorter the cracks, the greater the material's resistance to them. And the longer the cracks - the weaker the material's resistance to them. The difference in the fracture energy between the two states is very significant, up to three-four times. "From this it can be understood that it is possible to design applications that are based on lightweight brittle materials where relatively short cracks form, and then the material's resistance to their progress will be stronger," explains Prof. Sherman. The curve of the crack speed, V, versus the expended energy G0, in models with variable length. Short cracks show higher fracture energy.
It was also found that the profile of the cracks has a certain curvature, which led Prof. Sherman to develop an atomic model (called kinks, similar to the progression of a dislocation line in materials) that explains the relationship between the macroscopic properties of the fracture (energy for the fracture) and the breaking of the bond between the atoms on the cracks. This model clarifies the reasons why the energy content for the fracture of the cracks changes.
Prof. Sherman's group also uses a scanning tunneling microscope (with which surfaces can be examined at the atomic level), to examine the atomic structure of cracks in samples that have already broken. "In experiments such as these I can see how the progress of the cracks determines the way of breaking the interatomic bond in the material, forming atom after atom. This is how I actually understand the progress of the cracks qualitatively and quantitatively, at the microscopic and macroscopic level, and can explain precisely what the relationship between the material and the cracks is and why the degree of its resistance changes with their length."
In the next step, with the support of the National Science Foundation, Prof. Sherman and his team plan to do similar experiments with other materials, including brittle polycrystalline ones, such as glass and quartz.
Life itself:
Prof. Dov Sherman, married + three children, lives in Tel Aviv. In love with cracks since childhood ("When I was a student, my mother asked me what I was doing in the laboratory and I told her that I was breaking things. She mentioned that already at the age of one and a half I was hitting plates and glasses and testing their shattering. In short, you can say that I am the oldest experimenter in the field"). In addition, he likes to listen to classical music and travel with friends in Israel and around the world.
