The molecule that carries with it a message of regeneration
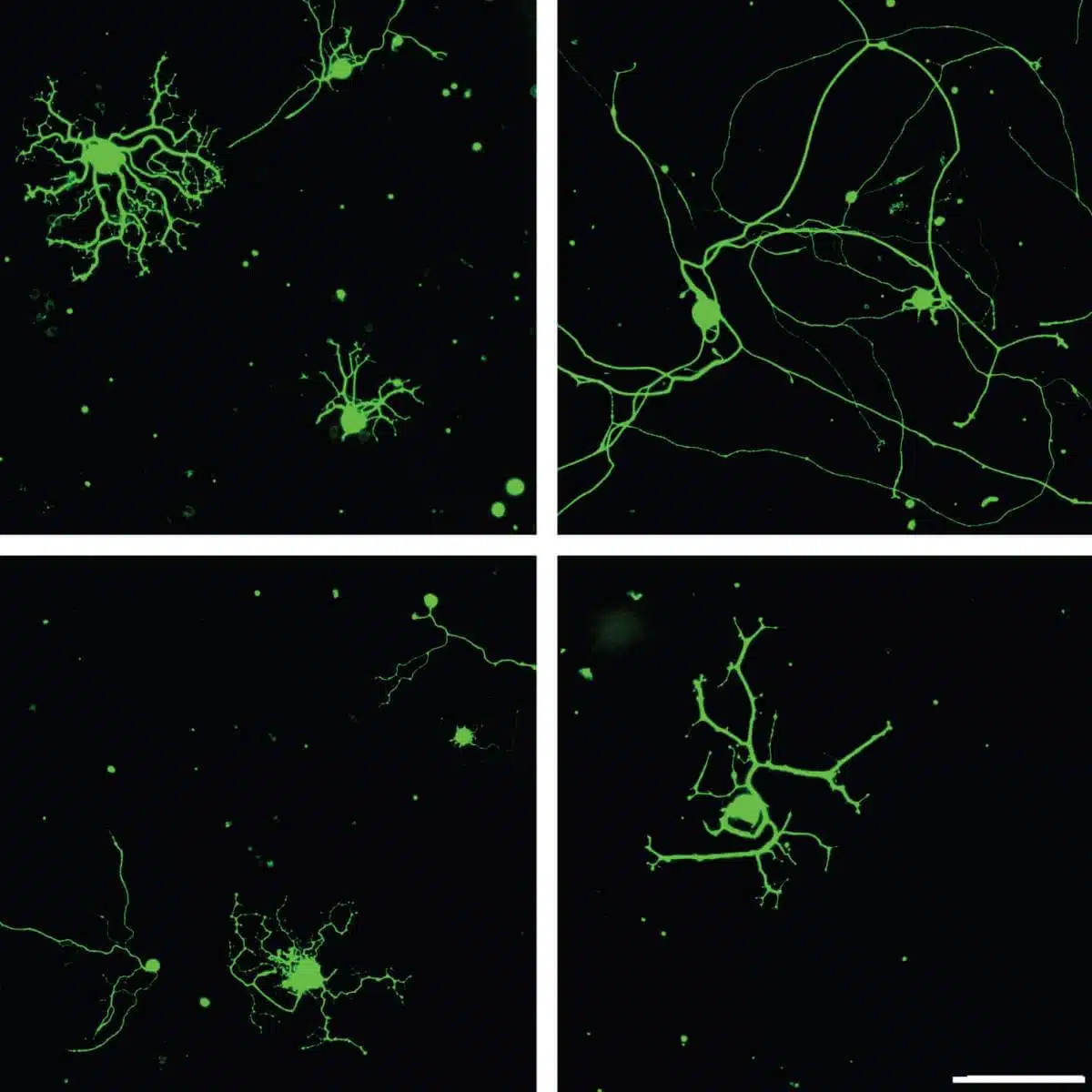
Sensory neurons of the peripheral nervous system in culture. After injury (right column) the neurons that express the protein PTBP1 (top row) regrow their branches much better than neurons with reduced expression of PTBP1 (bottom row)
A new study by Weizmann Institute of Science scientists reveals that the protein, which until now has only been observed during embryonic development, has a key role in the regeneration of mature nerve cells of the peripheral nervous system. When embryonic cells differentiate into adult cells, the composition of the proteins they produce changes. In the case of nerve cells, it was commonly thought that a decrease in the levels of a protein called PTBP1 in embryonic cells is a key part of their transformation into mature cells of the nervous system.
Previous studies have even shown that upon completion of the differentiation process and the transformation of the embryonic cells into mature nerve cells of the central nervous system, the production of the protein stops altogether. In recent years, these findings have led research groups to try to reproduce this process in the laboratory: to lower the PTBP1 protein levels in cells that are not nerve cells and thus produce mature nerve cells from them. The hope was that it would be possible to apply this method to patients with neurodegenerative diseases and to produce new nerve cells for them.
However, in a new study, led by the post-doctoral researcher Dr. Stephanie Alber and the doctoral student Pierluigi Di Matteo from the research group of Prof. Michael (Mike) Feinzylver in the Departments of Biomolecular Sciences and Molecular Neurobiology at the Institute, it was surprisingly discovered that the PTBP1 protein is expressed not only in unborn embryonic cells underwent differentiation, but also in mature neurons - although not neurons of the central nervous system, but yes of the peripheral one. The researchers discovered this completely by accident, while studying processes in the sciatic nerve of mice, nerves that leave the spine and reach the foot. The original experiment carried out by the researchers was aimed at finding molecules that regulate the rate of production of another protein called KPNB1 - "postcarriage" - responsible for carrying most of the messages transmitted from the distant extensions of the nerve cells to the cell nucleus. Among its other functions, the KPNB1 protein is responsible for signaling to the cell nucleus that a nerve branch is injured, so that the cell can regenerate it.
However, before the mail car can set off and carry messages from the branches to the nucleus, the cell is required to launch messenger RNA molecules that contain the "recipe" for the production of the mail car in the opposite path (from the nucleus to the branches). On the way or when arriving at the destination, other molecules may bind to the messenger, delaying or speeding it up and thus controlling the rate of production of the mail car. The surprising discovery in the new study was that not only was PTBP1 present in the mature cells, but it also bound tightly to the messenger RNA of the mail car. But does and how does it affect its production and the regeneration of nerve cells?
"Tens of millions of people around the world suffer from diseases that lead to the degeneration and death of nerve cells in the brain. In order to understand why the central nervous system fails in its attempt to regenerate after an injury, we must first understand how the peripheral nervous system succeeds in this." To answer this question, the scientists followed the response of the nerve cells to the injury and noticed that after three days the levels of PTBP1 in the cell began to rise and within A week they reached record levels. With the increase in PTBP1 levels, the researchers noticed that the nerve cell extensions begin to regenerate. Sequencing the messenger RNA molecules that bound to PTBP1 after the injury revealed that the protein binds not only to the messenger RNA molecules of the mail car, but also to other proteins that play a role in nerve regeneration.
Dr. Stephanie Alber To continue investigating the activity of PTBP1 in adult cells, the researchers removed it from the cells through genetic engineering, and showed that as a result, the regeneration of nerve cells of the "alarm receptor" type - cells whose role is to transmit a sensation of pain in response to a harmful stimulus that could damage the tissue - was impaired. The scientists also examined whether silencing the gene had additional effects and discovered that it increased the sensitivity to mechanical stimuli and heat. In an attempt to deepen the understanding of how PTBP1 affects the regeneration of nerve cells, the researchers examined whether it also affects another protein, RHOA - an important "control switch" in the process of differentiation and regeneration of nerve cells. When the RHOA protein is produced at high levels, it acts as a sort of off switch that inhibits cell growth. The researchers discovered that PTBP1 suppresses the production of the control switch in the extensions of the nerve cells, thus enabling their growth and regeneration. These findings strengthen the possibility that the production of PTBP1 in peripheral nerve cells is what enables their efficient regeneration, unlike in the central nervous system.
"Tens of millions of people around the world suffer from diseases that lead to the degeneration and death of nerve cells in the brain," says Prof. Feinzylver. "In order to understand why the central nervous system fails in its attempt to regenerate after an injury, we must first understand how the peripheral nervous system manages to regenerate. In this study, we identified a key protein in the regeneration process of the peripheral nerves, and now it will be possible to test whether and how it affects the limited regeneration capacity of the central nerves."
Dr. Agustina di Physio, Philip Freund, Dr. Nicholas Panagiotis, Dr. Letizia Marbledi, Dr. Ella Doron-Mendel, Dr. Natalia Okladnikov and Dr. Ida Rischel from the Departments of Biomolecular Sciences and Molecular Neurobiology at the Institute also participated in the study; Dr. Rinat Nebo from the department of biomolecular sciences at the institute; Matthew D. Zdardzinski, Irene Della Costa, Dr. Song Joon Lee, Prof. Pavitra K. Sehu, and Prof. Jeffrey L. Twiss of the University of South Carolina; Dr. Katelyn P. Medzhirtsky and Prof. Alma Burlingheim from the University of California, San Francisco; Dr. Ricky Kawaguchi and Prof. Giovanni Coppola from the University of California, Los Angeles.
More of the topic in Hayadan:
