The idea of capturing carbon dioxide or converting it to other substances in order to combat climate change is not new. However, simply storing carbon dioxide in a certain compound may lead to its leakage into the environment * The money from the sale of the carbon nano fibers will go to the continuation of the process and for the first time create a facility that has a negative carbon footprint
[Translation by Dr. Moshe Nachmani]
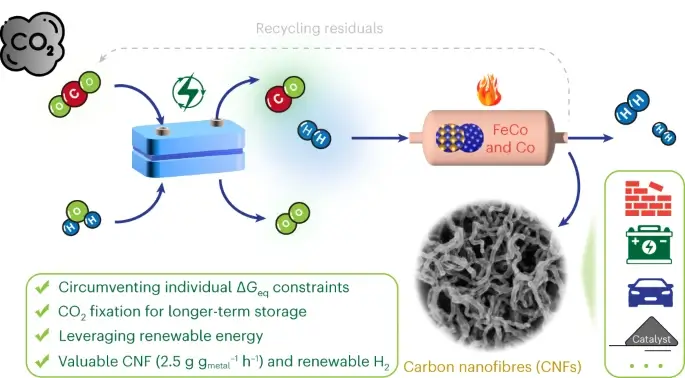
Scientists from the US Department of Energy and their partners from Columbia University succeeded in developing a method capable of converting carbon dioxide, a common greenhouse gas, into carbon nanofibers, a material with diverse unique properties and many future applications and therefore of economic value, while removing it from the air.
The researchers' approach was to use a coupling of electrochemical and thermochemical reactions that occur at relatively low temperatures and moderate pressure. As the scientists describe it in the scientific journal Nature Catalysis, this approach could successfully capture carbon in solid form and reduce its amount in carbon emissions. "The carbon nanofibers can be incorporated into cement to make it even stronger," said Jingguang Chen, a professor of chemical engineering at Columbia University who led the research. "With this method, it will be possible to trap the carbon inside the cement for at least a period of fifty years, and maybe even more. Until then, we hope, humanity will be diverted to use mainly renewable energy sources, those that do not emit carbon." As a bonus, the process also creates hydrogen gas, a promising alternative fuel, which when used, creates zero emissions (of carbon gases).
The idea of capturing carbon dioxide or converting it to other substances in order to combat climate change is not new. However, simply storing carbon dioxide in a certain compound may lead to its leakage into the environment. At the same time, many conversion processes of carbon dioxide into another material ultimately create carbon-based materials or fuel that requires direct and immediate use, use that emits carbon dioxide back into the environment. "The innovation in our research is that we convert carbon dioxide into a valuable substance, but one that is in a solid form, a particularly useful form," adds the lead researcher.
Such solid carbon materials, including carbon nanotubes and nanofibers, have valuable properties, including strength as well as electrical and thermal conductivity. However, it is not simple to get carbon from carbon dioxide and incorporate it into these chemical structures. Such a direct process, for example, is a process based on high heat of about one thousand degrees Celsius. "Such a process is unrealistic for capturing carbon dioxide on an industrial scale," explains the researcher. "In contrast, we found a process that takes place at a temperature of only four hundred degrees Celsius, a process that is much more practical, using a temperature available in the industry."
The uniqueness of the innovative approach was in the splitting of the reaction into stages and the use of two types of catalysts - substances that accelerate the approach of two reacting substances between them. "If you split the reaction into several sub-reactions, you can consider using different energy inputs and catalysts as part of the overall reaction," explains the lead author of the paper (Zhenhua Xie). The scientists started with the insight that carbon monoxide is a much better starting material than carbon dioxide for the preparation of carbon nanofibers (CNF). In the next step, the scientists tried to find the most efficient way to produce carbon monoxide from carbon dioxide.
A previous study by the same research group encouraged the researchers to use a commercially available palladium-on-carbon electrocatalyst. Electrocatalysts accelerate chemical reactions based on an electric current. In the presence of flowing electrons and protons, the catalyst manages to split both carbon dioxide and water to form carbon monoxide and hydrogen. In the second step, the scientists used a heat-activated thermocatalyst consisting of an iron-cobalt matrix. The catalyst is active at temperatures of four hundred degrees Celsius, significantly less than required for the direct reaction of converting carbon dioxide into carbon nanofibers. They also found that adding a small excess amount of metallic cobalt significantly boosted the production of the carbon nanofibers. "By coupling an electro-catalyst and a thermo-catalyst, we utilize a combined process to achieve results that could not be achieved with separate processes," says the lead researcher.
In order to reveal the details of how the catalysts work, the scientists conducted a wide range of experiments, including computer modeling, physical and chemical characterization, use of lasers and microscopes. "We were able to see the details of the various structures in order to determine the stable states of the catalyst during its activity under the reaction conditions," explains one of the researchers. "We observe the active sites and try to understand how these sites bind the intermediate forms in the reaction. "By determining the energy barriers, or transition states, from one phase to another, we learn exactly how the catalyst functions during the reaction." "According to our research, the iron-cobalt sites within the matrix help in splitting the carbon-oxygen bonds of the carbon dioxide. In this state, the carbon atoms are available to function as a source for the creation of carbon nanofibers. In the next step, the excess amount of cobalt encourages the creation of carbon-carbon bonds that connect to the carbon atoms," explains the researcher. The microscope images showed that as the carbon nanofibers form, the catalyst moves up and away from the surface, which makes it easier to recycle the metallic catalyst," explains the researcher. "We used acid to leach out the metal without destroying the carbon nanofibers, so the metal catalysts can be recycled and used over and over again," said the researcher.
The simplicity of the catalyst's recycling, the commercial availability of the metal catalysts, and the relatively mild reaction conditions all contribute to advantages related to energy consumption and other process-related costs, the researchers say. "For practical applications, the following two factors are really important - the mapping of the carbon dioxide footprint and the recyclability of the catalyst," said the lead researcher. "Our technical results and the other tests we conducted show that our method opens an efficient window to convert carbon dioxide into valuable solid carbon products while generating renewable hydrogen." If we can activate these reactions by renewable energy, then our findings will be truly "carbon negative", while creating new opportunities for effective reduction of carbon dioxide."
More of the topic in Hayadan:

4 תגובות
A comprehensive and interesting article. Since I have been following the articles of the signatory of this article for years, I appreciate that the use of large language models greatly improves the quality of the articles.
The science site is exactly the opposite of a missionary site, it promotes rationality, and no religion is rational and certainly not cults so this is a refutation of your claims. As for being anti-Israeli, if you had read countless articles about what is happening in universities in Israel, including Bar Ilan and Ariel, you would understand that I am not anti-Israeli. Unless, in your eyes, anti-Israeli is anyone who opposes the takeover of Israel by dark elements, so to speak, in the name of the Jewish religion. There is no need to explain now what the problem is with the state having to take high interest loans to finance the war and instead of cutting it doubles the payment to yeshiva students and other ultra-Orthodox occupations to NIS 1.7 billion.
Cloud Continents: Terraforming Venus
Efficiently by Means of a Floating Artificial
Surface
Alex R. Howe
https://arxiv.org/pdf/2203.06722.pdf
Now you just need to connect them
Caution this is a missionary site. or anti-religion, inappropriate for the Jewish people