An international team of researchers has discovered a new family of magnetic superhalogens - a type of extremely stable atomic clusters that could be used to advance the field of materials science through the preparation of salts with magnetic and oxidation properties that did not exist before.
The discovery, about which an article was published in the scientific journal applied Chemistry, was based on theoretical work done by researchers from several universities in the USA (Virginia Commonwealth University, McNeese State University) and China (Peking University) and on practical experiments conducted at Johns Hopkins University.
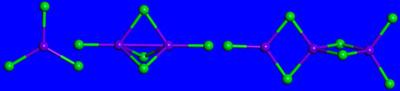
Unlike normal superhalogens, which consist of a metal atom in the core and which is surrounded by halogen atoms, the new magnetic superhalogens consist of halogen-metal groups in the core to which other surrounding halogen atoms are attached.
The new chemical forms, called "magnetic superhalogens", mimic the chemistry of the halogen group - a family of elements in the periodic table that includes the elements fluorine, chlorine, bromine, iodine and astatine. The meaning of the word "halogen" is "salt-maker", this is due to the fact that when one of these atoms is joined to a metallic atom, salt is obtained (home table salt consists of one chlorine atom and one sodium atom).
Practically, the new mass prepared by the researchers is symbolized by the general formula MnxCl2x+1, When x represents an integer (1, 2, 3 and so on), Mn represents the metal atom manganese and Cl represents an atom of chlorine, and when the manganese and chlorine atoms are bonded together and serve as the core of the aggregate, and another single chlorine atom is bonded to them. The manganese atoms have a large magnetic moment and are therefore responsible for the fact that the aggregate as a whole is magnetic.
"Now, additional unknown magnetic superhalogens can be designed and synthesized by changing the metal atom from manganese to other transition metal atoms, and by replacing the chlorine atom with other halogen atoms. In addition to their use as oxidizing agents, being magnetic allows us to make a whole new family of salts," said lead researcher Puru Jena, a professor of physics at the University of Virginia.
According to the researcher, superhalogens are similar to halogens in that they easily form negative ions, but their ability to attract electrons is much greater than any other known halogen. Negative ions are useful as oxidants, for cleaning the air and releasing the mood-elevating substance serotonin.
"The superhalogens are able to perform the same tasks as the halogens - only better," says the researcher. "The ability of superhalogens to store large amounts of fluorine and/or chlorine atoms could also be useful in the fight against harmful biological substances. In addition, due to their high electronic affinity, they will be able to lead to the development of new chemistry," notes the researcher. The same research team discovered last October a new family of materials, called "hyperhalogens", which themselves serve as building blocks for the superhalogens. These materials could have many and varied applications in industry. The news about the study
