A challenge that has occupied chemists for decades - the preparation of stable and useful radical molecules - has been solved in research conducted at the Technion
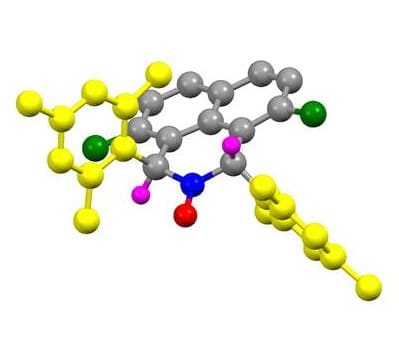
A challenge that has occupied chemists for decades - the preparation of stable and useful radical molecules - has come to its solution in research conducted at the Technion. The research was carried out by the research group of Associate Professor Alex Spielman from the Shulich Faculty of Chemistry at the Technion and Dr. Mark Iron from the Weizmann Institute assisted in the calculations that explain the stability achieved. The results of the study were published Friday (February 6, 2015) in the journal Nature Communications.
Radical molecules are molecules that contain an odd number of electrons. Electrons tend to organize in pairs and thus create an energy balance, therefore in the aforementioned situation - an odd number of electrons, and one unpaired electron - there is an energy imbalance. To achieve energy balance, the radical molecule requires an additional electron, so it tends to react quickly to other molecules, meaning it is unstable.
Despite this rule - according to which radical molecules are unstable - stable radicals exist. In 1900, a stable organic radical with a tertiary structure, triphenylmethyl, was discovered for the first time by Professor Moses Gomberg from the University of Michigan. This historic discovery led to the discovery of other stable radicals. In 1960, TEMPO - another very stable organic radical - was discovered by Lebedev and Kazarnowskii. These molecules are currently used in the pharmaceutical plastic industry, in rubber production as well as in medicine, agriculture, biochemistry and measuring distances in biological systems such as DNA.
However, the known stable radicals have many disadvantages, including a high production cost and a spatial structure that limits their use.
The new molecule developed by associate professor Spielman - stable α-hydrogen nitroxyl radical - may bring about a dramatic change in this field. It is a stable radical with a more 'usable' spatial structure. At the end of the article, Associate Professor Spielman points out that this molecule is particularly attractive in the context of future applications in catalysis and in the context of biological studies. "In fact, a path is paved here for many applications in catalysis, biology and medicine."
Alex Spielman, born in Denmark, came to Israel at the age of 18 as a volunteer at Kibbutz Hamadia. After returning to Denmark and completing a master's degree in medicine there, he returned to Israel, completed a doctorate at the Weizmann Institute and a post-doctorate at ETH Zurich, and in 2009 joined the Technion as a faculty member in the Faculty of Chemistry.
During his advanced degrees, as well as in his post-doctorate, Spielman engaged in the organic synthesis of natural substances, that is, in the preparation of natural substances in the laboratory and in the study of their biological activity. When he arrived at the Technion he decided to return to basic science.
"This is my strategic view today," he explains. "I want to start with a basic scientific discovery, and from there develop it to synthesis and application. Chemistry is a great and fascinating field that requires a lot of creativity from you. There is something very satisfying about developing a new idea and bringing it to fruition."
