Turning lead into gold is an impossible task, however, a similar type of "alchemy" is not only possible, but even economically efficient. Several combinations of elemental atoms have an electron pattern that mimics that of other elements
Turning lead into gold is an impossible task, however, a similar type of "alchemy" is not only possible, but even economically efficient. Three researchers from Penn State University (Pennsylvania State University) have shown that several combinations of elemental atoms have an electron pattern that mimics that of other elements.
According to the lead researcher, A. Welford Castleman, professor in the departments of chemistry and physics, "the findings could lead to obtaining cheaper materials for common applications such as new sources of energy, methods to reduce pollution and catalysis, on which industrial companies depend heavily for chemical processes."
The researchers also showed that the atoms identified as participating in these mimic patterns could be predicted simply by looking at the periodic table. The research team used advanced experiments and theories to quantify these new and unexpected findings. "We get a completely new perspective on the periodic table," says the lead researcher. The findings were published in the scientific journal Proceedings of the National Academy of Sciences.
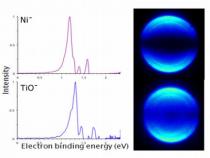
The research team used a spectroscopic method known as "photoelectron imaging spectroscopy" (photoelectron imaging spectroscopy) to examine similarities between titanium monoxide and nickel, between zirconium monoxide and palladium, and between tungsten carbide and platinum.
"Photoelectron microscopy measures the energy required to remove electrons from different energy levels of atoms or particles, while taking flash photographs of these "expulsion" events using a digital camera," explains the researcher. "The method allows us to determine the binding energies of the electrons as well as directly observe the nature of the orbitals in which the electrons were located before they were removed. We found that the amount of energy required to remove electrons from the titanium monoxide is the same as the amount of energy required to remove electrons from an atom of the nickel metal. The same is true for the pairs of systems - zirconium monooxide versus palladium, and tungsten carbide versus platinum. The key to the results lies in the fact that all pairs are isoelectronic forms, which are atoms with the same electronic configuration."
The researcher points out that in these cases the term isoelectronic refers to the number of electrons found in the outermost level of the atom or atom.
The researchers examined the images obtained from the photoelectron spectroscopy data. The patterns of the bright spots in the image, which correspond to the energy of the electrons emitted during their removal from the outer levels of the atom, looked very similar between the three pairs of forms examined in the study. Similarly, the graphical displays of the energy levels looked very similar between the three pairs of forms; Theoretical calculations also showed a high correspondence between the energy levels of the pairs of trusons.
The researcher explains that titanium monoxide, zirconium monoxide and tungsten carbide are essentially superatoms of nickel, palladium and platinum, respectively. Superatoms (Wikipedia entry) are aggregates of atoms that exhibit some of the properties of elementary atoms. Previous research conducted in the same laboratory examined the very idea of superatoms. One of these experiments showed that a cluster of thirteen chemran (aluminum) atoms behaves like one iodine atom. Adding a single electron to this system of chymran atoms caused the aggregate to behave like an atom of a noble gas. In addition, the study showed that a cluster of fourteen atoms of chemran has an activity similar to that present in an atom of an alkali metal ore.
Now, scientist Castleman's new research advances the idea of a superatom to an entirely new level and provides a solid quantitative basis for this relatively new concept. "It looks like we're able to predict which combinations of atoms of the elements will be able to mimic atoms of other elements," he says. "For example, by looking at the periodic table it is possible to predict that titanium monoxide will form a nickel superatom. Just start with titanium, which contains four electrons in its outermost level, and move six elements to the right in the periodic table since an oxygen atom has six electrons in its outermost level. The element you arrived at is nickel, whose ten electrons in its outermost level make it isoelectronic against the combination of titanium and oxygen. We thought to ourselves that this finding must be a rare coincidence, so we continued and tested it with other atoms and found that the same pattern of action emerges."
The researcher says that he does not know if this pattern will repeat itself throughout the periodic table or if it will be limited to only a part of it. Now, the research team is looking at atoms of the transition metals. In the future, they plan to take the research one step further and test whether the chemical properties of the superatoms are similar to the individual atoms that make them up. "Today, platinum is used in almost all catalytic converters in vehicles, but it is a very expensive material," says the lead researcher. "In contrast, tungsten carbide, which is an "imitation" for platinum, is cheap. It would be possible to save significantly on economic costs if the manufacturers of catalytic converters could use tungsten carbide instead of platinum. Similarly, palladium is used in certain combustion processes, and can be imitated by zirconium monooxide, which is five hundred times cheaper than it. Our new findings are fascinating both from a scientific point of view and from a practical economic point of view."
The news from the university

8 תגובות
Does it have to be done at very low temperatures to create a superatom? And if so, was the research done so that first they reduced the temperature to create a superatom and then created other atoms from that atom by combining electrons to a superatom?
The method allows us to determine the binding energies of the electrons as well as directly observe the nature of the orbitals in which the electrons were located before they were removed. The amount of energy required to remove electrons from the titanium monoxide is the same as the amount of energy required to remove electrons from an atom of the nickel metal. The same is true for the pairs of systems - zirconium monooxide versus palladium, and tungsten carbide versus platinum. The key to the results lies in the fact that all pairs are isoelectronic forms, which are atoms with an electronic configuration
This research seems important and fruitful, maybe at the level of part of the Nobel Prize, who knows.
I did not understand to what extent the 'imitation' achieved by an accumulation of atoms is comprehensive. That is, will there still be differences in the behaviors or properties of the 'original' material
and imitation, and if so - what kind?
Super electron, you are confused: the atoms are not alike but the protons, neutrons and electrons that make them up.
I didn't understand where the problem was.
super electron
You can't say that what you were taught is bullshit...it's the basis of the basis. Just like you can't expect string theory to be taught in high school physics majors. The new findings do not contradict the basic knowledge, but are simply a very advanced extension of it.
This study is more strange than interesting!
Since when are there atoms of elements? Do the atoms know about it? Aren't the atoms all supposed to be the same?
I'm not asking, I'm getting angry, because once again they sold us nonsense and now they say listen to this is the truth and not what we have been telling you all these years, and they even dare to call it exact science!!
Shu de atom on? So when will they discover super metals in "excitement" followed by super metals and mega super!
How do people quickly eat all the bullshit without even asking a small clarifying question!!
Indeed amazing! But without disrespecting the chemists, how come they didn't think of this before?
Appreciate them