A team of researchers led by Associate Professor Mittal Landau from the Faculty of Biology at the Technion deciphered a new structure of a protein that was not known until now. The article published in the prestigious scientific journal Science Reveals for the first time an amyloid fiber with which the virulent bacteria "Staphylococcus aureus" attacks the organism's cells and its immune system. The scientific breakthrough is expected to lead to the development of a dedicated antibiotic drug that will deal with the bacteria more effectively.
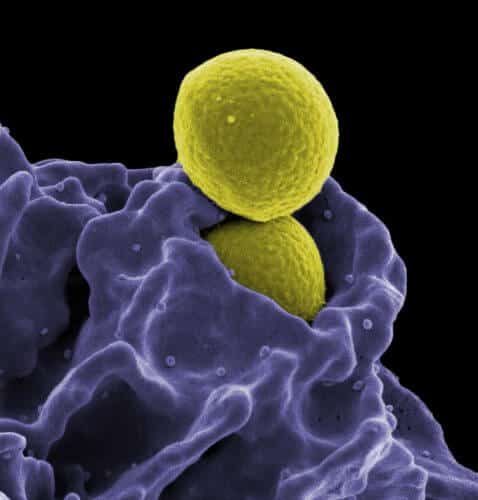
Staphylococcus aureus, one of the most violent bacteria known today, attacks the cells and the immune system of the host organism, partly through unique fibers it secretes. This is according to a study led by Associate Professor Mittal Landau from the Faculty of Biology at the Technion. In her estimation, the discovery is expected to lead to the development of a dedicated antibiotic that will damage the fiber and thus allow the cells of the immune system to deal with the bacteria effectively.
The research published in the journal Science was carried out by researchers at the Landau laboratory: PhD student Einav Tayeb-Fligelman, the director of the laboratory Dr. Orli Tabechnikov, Asher Moshe and Dr. Orit Goldschmidt-Tran. Researchers from the particle accelerator in Grenoble (France) and the University of California Los Angeles (UCLA) also contributed to the work.
Staphylococcus aureus is a particularly aggressive bacterium that was discovered at the end of the 19th century in Scotland and was used by Alexander Fleming in the famous culture in which penicillin was discovered. This bacterium is resistant to many strains of antibiotics and is responsible for some of the infection cases that occur in hospitals and in the community. Its Latin name, Staphylococcus aureus (golden grape cluster), derives from its cluster-like shape.
In the article, which also presents the first crystalline structure of its kind at atomic resolution, a weapon that helps the bacterium was revealed: an amyloid fiber of a type that was not known until now. Amyloids, proteins notorious for their link to neurodegenerative diseases such as Alzheimer's and Parkinson's, form protein fibers - a kind of spider's web - characterized by a very ordered and stable structure. This stability allows them to endure extreme conditions in which normal proteins do not survive, and one of the famous examples of this is the "mad cow" epidemic that broke out in England in 1986. According to Associate Professor Landau, "this disease surprised the scientific community because its cause was neither a virus nor A bacterium but a protein called a prion, which has an amyloid structure. Suddenly it became clear that protein can also pass between organisms, and due to its stability it infected humans through eating infected meat - that is, it did not break down during the stages of meat processing, cooking and digestion."
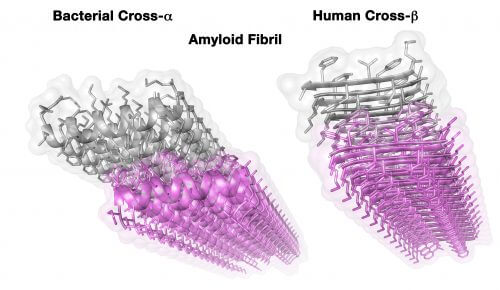
The "mad cow" prion protein, like all amyloids discovered so far, belongs to a group with a structure called cross-ß. In the current study, an amyloid with a completely new structure was discovered, which was named cross-α. "We knew we had something unique in our hands, but only after several trips to particle accelerators in Grenoble and Chicago were we able to verify that it was a new type of amyloid. In order to publish the discovery in Science, a lot more work was required, but from the first moment it was clear to us that this was a very dramatic discovery that represents a paradigm shift."
Targeted antibiotics and a first glimpse into a new world of amyloids
Associate Professor Landau estimates that the discoveries published now may lead to the development of types of antibiotics based on a new mechanism of action. This mechanism will damage the protein (amyloid) and thus neutralize at least one of the important weapons of the violent bacteria. In her estimation, since this antibiotic will not eliminate the bacteria but will only increase its vulnerability, it will not lead to the rapid development of bacterial resistance to it. "Resistance to antibiotics develops in bacteria following evolutionary pressure - natural selection leads to the development of bacteria that antibiotics fail to kill. If we reduce the pressure on the bacterium, and do not harm it but only in its violent aspects, it is possible that resistance to antibiotics will not be in a hurry to develop."
Regarding the way forward, Associate Professor Landau clarifies that drug development is usually done outside the walls of the academy due to the high cost of the process. "However, we can give drug developers scientific knowledge that will speed up and reduce the development process. The current discovery - deciphering the structure of cross-α amyloid - is a leap forward in such a process. Now our challenge is to find the substance that will inhibit cross-α and thus disarm the bacteria. In the scientific aspect, there is an important lesson here: thinking outside the box opens new doors to the world of amyloids. Deciphering new structures may lead to insights related to both neurodegenerative diseases and 'good' amyloids, which participate in natural defense against infections. These findings will enable the development of measures to protect against toxins in bacteria and fungi."
Thanks to the research infrastructure
Associate Professor Mishna Landau completed a bachelor's degree in pharmacy at the Hebrew University and two degrees at Tel Aviv University - a master's degree in neurobiochemistry and a third degree in structural bioinformatics. After her doctorate, she went on to do a post-doctorate at the University of California Los Angeles (UCLA), where she specialized in X-ray microcrystallography and amyloids related to Alzheimer's disease. "At the end of the post-doctorate," she says, "I chose the Technion partly because of the excellent research infrastructures that exist there. The Center for Structural Biology, which was established on the initiative of the Nobel laureate in chemistry, Professor-Researcher Aharon Chechenover with an investment of about 5 million dollars, has an excellent world-class infrastructure that allows solving protein structures at atomic resolution." Over the years, Prof. Mishna Landau has won many scholarships and grants, including the Marie Curie grant, the Center for Excellence in Israel grant (ICORE, the Alon scholarship, and grants from the Israel-USA Binational Foundation, the National Science Foundation and the Israel-Germany Foundation (DIP).

2 תגובות
Avi. Keep the comments to yourself. People died from this germ. Give respect to the research that will save many people.
Amazing, so beautiful and smart that I want to cry.