A small molecule that inhibits the spread of cancer metastases - an original development by researchers at the Technion, the Netherlands and the UK
Researchers at the Technion, the Netherlands and the UK have developed a molecule that inhibits the spread of cancer metastases. In an article in the journal PNAS, the researchers demonstrate, in preclinical experiments, the effectiveness of the molecule in inhibiting lung cancer, breast cancer and blood cancer.
Professor Israel and Ludavsky from the Rapaport Faculty of Medicine and his colleagues at Leiden University in the Netherlands and York University in the UK are responsible for the invention. In light of the positive results, the researchers submitted a request to register the molecule as a patent, and they estimate that the findings will pave the way for the development of the molecule towards clinical applications.
Cancer metastases, which are the main cause of death from cancer, occur when cancer cells migrate from the primary tumor to other sites in the body. Their formation depends on the ability of the cancer cells to break away from the primary tumor, invade through the blood vessels into other tissues and settle in a new organ.
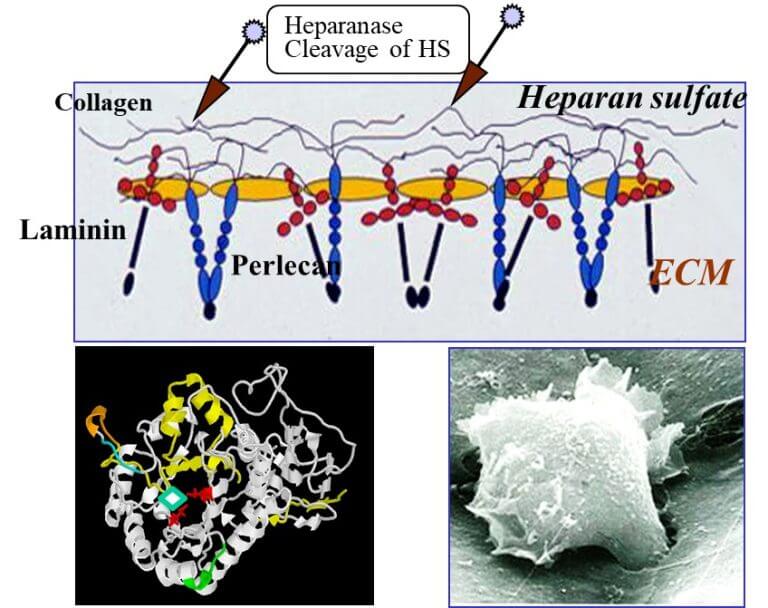
One of the natural barriers of the body's tissues against cancer invasion is a sugary substance called heparan sulfate (HS). This substance is found in the extracellular matrix (ECM) which gives the body tissues shape and support and maintains their integrity. The problem is that during evolution, cancer cells have developed mechanisms that change the extracellular tissue around them, recruit it for their needs and penetrate through it more easily.
Top: Schematic of the intercellular medium. Bottom left: the structure of the hyperenase and presentation of the active site in the enzyme (in red) and the inhibitory molecule (arrow). Bottom right: tumor cell infiltration
One of the weapons of the cancerous tumor is heparnase - an enzyme that cuts the heparan sulfate and thus changes the structure of the extracellular material and the tissue around the primary tumor and also causes the release of growth-promoting proteins. In this way, the farnase accelerates the growth of the primary tumor, encourages the formation of blood vessels that feed it and provides routes of spread to additional tissues and the formation of metastases.
This is the background for the development of the paranase inhibitors as a significant research and medical goal in the search for anti-cancer treatments. Prof. Volodevsky and his team - Dr. Uri Barash, Dr. Neta Ilan and doctoral student Yasmin Kiel - who have been researching the enzyme farnase for many years, tested in the current study a farnase inhibitory molecule that is expected to suppress the said metastatic mechanism.
The new molecule, developed with Prof. Herman Overclift from the University of Leiden and their partners (L. Wu and G. Davies) at the University of York, binds to residues (amino acids) critical to the activity of the paranase enzyme in a stable and irreversible (covalent) manner and prevents it from attaching to the paran sulfate chains and cutting them. In this way, the extracellular medium maintains its structure and the migration of cancer cells and the formation of metastases are prevented.
According to Prof. Wlodavsky, "paranase inhibitors have already been developed in the past, but so far not a single one of them has been approved for clinical use, and we estimate that the new molecule will break through this barrier. In the study, we showed that our molecule binds in a specific and permanent (covalent) chemical bond to the active site in the enzyme and causes an irreversible inhibition, that is, creates a permanent barrier to the metastatic process. We estimate that further studies that will improve this molecule will lead to a significant change in cancer treatment."
More of the topic in Hayadan:
- Researchers have found a way to delay the development of mesothelioma cancer
- Cancer metastases sometimes form following successful chemotherapy treatment
- Lethal learning: a new interpretation of adaptive mechanisms of cancer cells
- Where are the medicines to treat Corona?
- Significant progress in research on the way to finding a cure against the corona including the variants
