The research was led by researchers from two faculties at the Technion and their colleague from the University of Tokyo
A new article published in-Nature Communications. Presents unique peptides with anti-cancer potential. The research was led by Prof. Ashraf Barik and the post-doctoral students Abhishek Saha and Ganga and Misty from the Shulich Faculty of Chemistry together with Prof. Navia Ayyub from the Faculty of Biology at the Technion and Prof. Hiro Suga from the University of Tokyo.
Peptides are short chains of amino acids. Unlike proteins, which usually contain many hundreds of amino acids, peptides contain at most dozens of such acids. The circular peptides that the researchers discovered bind specifically to chains of ubiquitin proteins - a protein that is usually used as a "death tag" for damaged proteins. The labeling of the damaged proteins leads to their being broken down in the proteasome, the garbage can of the cell. The discovery of the ubiquitin system led in 2004 to the awarding of the Nobel Prize in Chemistry to three researchers, two of them from the Rapaport Faculty of Medicine at the Technion: Research Prof. Aharon Chachanover and Research Prof. Avraham Hershko.
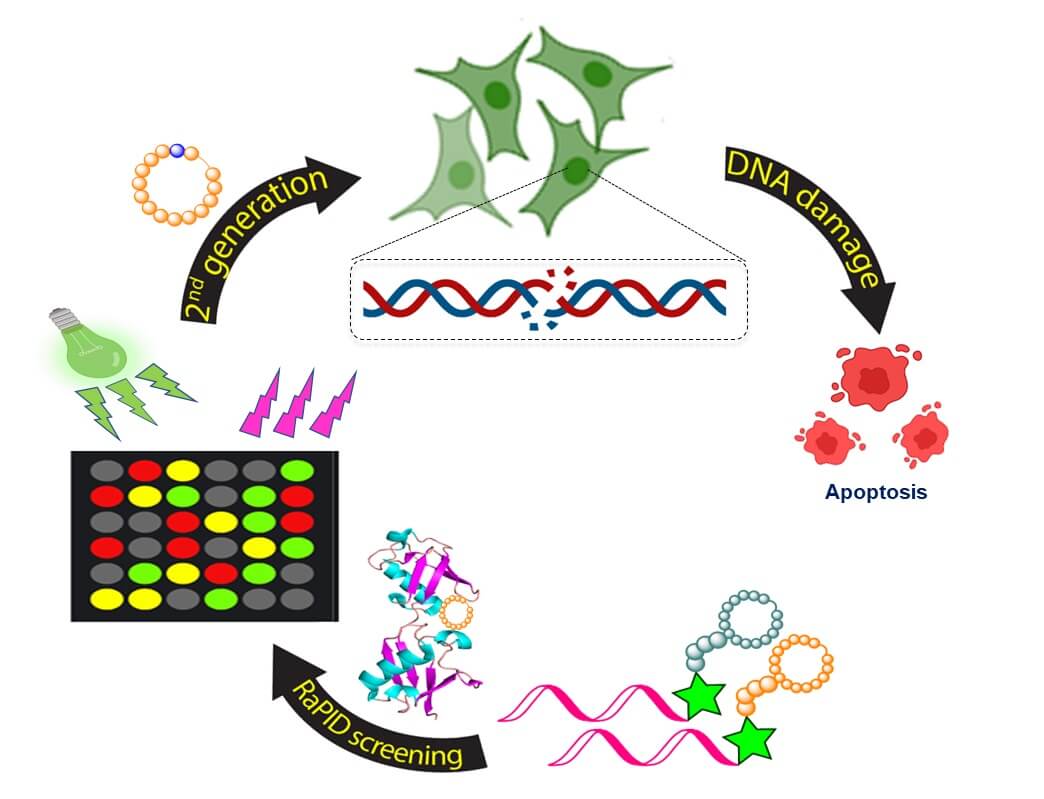
In recent years, it became clear that the activity of the ubiquitin system depends, among other things, on the point where the ubiquitin molecules bind in the chain. For example, binding to position 48 (K48) leads to the removal of proteins to the proteasome, while binding to position 63 (K63) in ubiquitin leads to the repair of damaged DNA.
In recent years, Technion researchers have developed a new approach to influencing the ubiquitin mechanisms: instead of interfering with the activity of enzymes that affect these mechanisms, they decided to try to directly intervene in the ubiquitin chain itself. From this approach, the researchers in a previous work developed ring peptides that bind to position 48 in the ubiquitin chains and thus Are driven from which lead to the breakdown of the damaged proteins. This disruption gradually leads to the death of the entire cell. In the same study, they hypothesized - and then showed - that when such a link is formed in a cancerous tumor, it kills the cancer cells and thus protects the patient. this discovery, Published in the journal Nature Chemistry In 2019, led to the establishment of a new startup company advancing the discovery towards clinical use.
In the current study, ring peptides were produced that bind to position 63 in ubiquitin - the leading position as mentioned for repairing damaged DNA. The researchers discovered that binding the peptides to this position disruptive the aforementioned repair mechanism and leads to the accumulation of damaged DNA and thus to cell death. Here too, when this attachment occurs in cancer cells, it destroys these cells. The researchers estimate that it is possible that this therapeutic strategy will be more effective than existing anticancer drugs that lead to the development of resistance against them.
Prof. Ashraf Barik He is the head of the Jordan and Iran Tarek Chair in the Shulich Faculty of Chemistry. He won many awards of excellence, including the Outstanding Researcher Award from the Israel Chemical Society and the prestigious ERC Advanced Grant.
