A system developed at the Technion shows unprecedented efficiency in producing hydrogen from water using solar energy
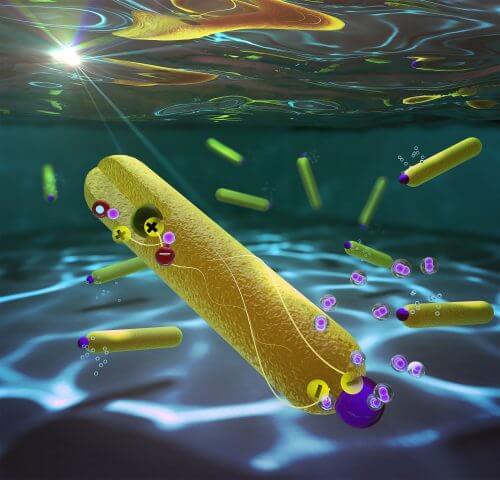
The journal Nano Letters reports on a dramatic turn in the field of hydrogen fuel production: 100% efficiency in the production process. The breakthrough took place in the laboratory of Associate Professor Lilach Amirav from the Shulich Faculty of Chemistry, and its essence: a significant jump from 60% to 100% - in the production of hydrogen fuel by breaking down water using solar energy.
Hydrogen fuel is considered a promising energy source mainly because it is produced from water, which is always available, and therefore does not involve the political and economic problems that characterize the traditional energy market; And because, unlike fossil fuels (gasoline, etc.), its use is non-polluting. This is why the US alone invests billions of dollars in programs such as the Hydrogen Fuel Initiative, which encourage the development of hydrogen production technologies. Furthermore, these photocatalytic processes, in which water is broken down into hydrogen and oxygen, are also relevant to other areas, including energy conversion and wastewater purification.
However, many constraints make it difficult to develop efficient photocatalysis processes that meet the following basic conditions: optimal absorption of solar radiation by the catalytic material; Effective decomposition of the water into oxygen and hydrogen without meeting them (which may lead to an explosion), and stability of the system so that it does not lose its effectiveness over time.

Now, as mentioned, the journal Nano Letters reports on a breakthrough achieved in the laboratory of Associate Professor Lilach Amirav, a faculty member in the Shulich Faculty of Chemistry and a member of the Russell Berry Nanotechnology Institute and the Grand Energy Program at the Technion. While most of the research effort in the world is done on the empirical path - trial and error with different materials and testing their effectiveness - in the laboratory of Prof. Mishna Amirav, catalytic materials are developed based on a precise characterization of the material at the individual particle level. This is how advanced artificial materials have been developed over the years that are optimally suited to the photocatalytic task. The new system presented in the current article proves that the effort paid off; This system, centered on a nanometer photocatalyst operating in a very basic environment, breaks all previous conventions regarding maximum utilization and produces 360,000 moles of hydrogen per hour per mole of catalyst.
The system is built from two semiconductors, arranged as a tiny nanometer particle (quantum dot) of one material, planted asymmetrically inside the other material which is shaped like a nanometer rod, and at the end of the rod is a platinum particle. The quantum dot has the ability to attract and store positive charges. When the system is immersed in water and exposed to sunlight, the nanorod absorbs photons (light) and releases charges. The electrons (negative charge) accumulate in the platinum particle, while the positive charges accumulate in the quantum dot. The physical separation between these charges is the key to the success of the system. The electrons are responsible for redox, the reaction that produces hydrogen from water. When a water molecule breaks, a positively charged hydrogen ion is released. When two such ions react with two electrons, on top of the platinum, they join together to form a hydrogen molecule. The efficiency of the result, as mentioned, is 100% efficiency in this process, which means that every two photons created a hydrogen molecule, without losses, a goal that was considered impossible to achieve until now.

10 תגובות
Maybe the theoretical conversion is 100% but the production is lower. In practical physics there is no 100%. But if the discovery produces such high salvages, even with precious metals it is economically worthwhile. The use of concentration reduces the reception area and reduces costs.
The assessment, as seen from the explanation, is that it is not possible to produce large areas to absorb light and therefore additional technologies must be used to implement the invention.
If the receivers are platinum they will never be cheap no matter how much time passes. On the contrary, the demand for the rare metal will rise and so will the price.
Receivers that offer 100% efficiency will eventually have the technology available at a low price. Nano electrodes in semiconductor growth, this is an expensive technology. Arsenic gallium semiconductors instead of expensive silicon.
An electric vehicle will run on hydrogen yes. The main disadvantage - hydrogen is a gas that takes up a huge volume compared to fuel. I don't see a small compressor that compresses the gas at car level.
Therefore - perhaps it is not a domestic technology but an industrial one. Solar farms.
I'm talking about the purchase cost even if one time. A very large amount of collectors will be needed to provide electricity to, say, a city. If the price is too high, they will prefer to use a less efficient technology. Which means there will be almost no use in the invention.
Don't forget the cost of desalinated water!
Sea water is always available!
I'm only interested in the cost of the technology, there are usually cost issues for materials that contain platinum. Of course it depends on the amount of platinum needed. In any case, according to what I've heard, the platinum is one of the reasons that hydrogen fuel cells are still very expensive.
It really sucks that the most needed materials are found in too small a quantity in nature
It is interesting if we can convert the conventional vehicle to hydrogen (as we will do with the Dumas gas).
(And regardless, I wonder if they will allow conventional cars to become autonomous)
((And really unrelated: I wonder if we will manage to annoy [or insult] our autonomous vehicle {which will be powered by hydrogen}))
In I-CORE there are not only young people. There are also young people.
Recently, the Technion has seen child professors, 30 plus years old, with a quantity of articles that challenges the professors with 30 years of experience at the Technion. This is an encouraging sign. Not because of the older ones - a productive person has the right to create even if the rate of his articles decreases, and the wisest among them have research groups. But there is no blocking because of the number of standards for young researchers.
For example, Professor Yoash LeBron of Electricity, and Professor Nathaniel Linder and the list is not short.
There is an organized program there called I-core Israeli Excellence - someone in the Ministry of Science woke up a few years ago and measured Israel's rate of progress compared to the surrounding countries and the world and saw a worrying picture.
As a result, centers of excellence were opened that find gifted doctoral students and give them what they would get abroad, a professorship based on the number of articles they published and not at the age of 60 as in the past. and doing research at a young age. The same goes for the Young Academy of Sciences, which is a blessed project. Not everything done in the Ministry of Science deserves condemnation, and when there is room for positive criticism, I give it. By the way, the Minister of Science Akonis, in matters of science improves his image towards seriousness.