The discovery may lead to the development of resistant artificial peptides that will be used in innovative treatments for infectious diseases
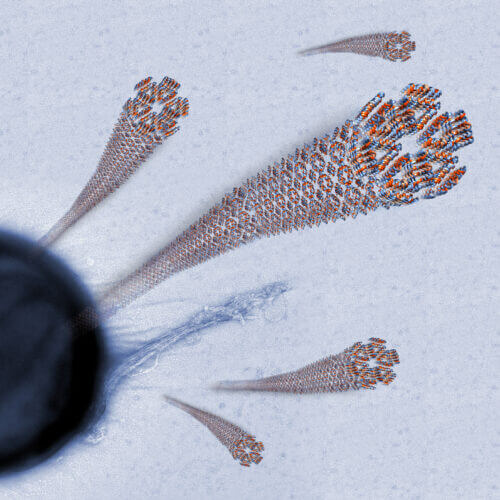
Technion researchers deciphered the atomic structure of an antibacterial peptide important in the human immune system and discovered that it forms fibers with a special structure. In their estimation, the findings may lead to the creation of similar artificial particles that will be used for targeted treatment of infections and even cancer cells. The discovery was published yesterday in the journal Nature Communications.
A peptide is a short chain of amino acids. Unlike proteins, which usually contain many hundreds of amino acids, peptides contain at most dozens of such acids. One of the important groups of peptides is the group of antimicrobial peptides (AMPs), which is the subject of the present study. This group plays a significant role in the innate immune system and helps it deal with various bacterial infections.
The Technion researchers, Prof. Mittal Landau and doctoral student Yitzhak Engelberg from the Faculty of Biology, focused in the current study on an active derivative of the antimicrobial peptide LL-37(17-29) which is found in the human body and helps it fight against various bacteria. In a study conducted at the Technion and particle accelerators in Europe, the two discovered that one of the means used by this peptide in killing bacteria is a unique protein fiber endowed with great stability and high heat resistance. The researchers mapped the form of self-assembly of the fiber - a long, ribbon-like structure that exhibits great stability in hostile conditions and adhesion to bacterial cells that allows it to attack the bacteria from point blank range.
Following the decoding of the unique structure of the fiber, Engelberg and Prof. Landau propose a new concept for the design of durable and stable artificial antibacterial peptides, which will replace antibiotic treatment in some cases. This is against the background of the fact that the antibiotic treatment, which has saved the lives of hundreds of millions in the last hundred years, causes the development of bacterial resistance that impairs its effectiveness. As we know, many of the deaths in hospitals today are caused by infection with antibiotic-resistant bacteria - resistance that developed as a result of the bacteria being exposed to high doses of broad-spectrum antibiotics.
Peptides are currently considered a great therapeutic promise since, at least in some cases, they are able to neutralize the population of harmful bacteria (pathogens) without causing the formation of bacterial resistance to treatment. Many research groups in the world are engaged in the development of peptides for medical purposes, and the engineering challenges are many: efficiency, selectivity, adaptation to body tissues (bioavailability), stability in storage (shelf life) and stability in the body after ingestion.
According to Prof. Landau, the current study is an important step in the realization of these goals. The atomic and molecular structure of the natural peptides, which was deciphered in the current study, will make it possible to produce similar structures that will serve as a skeleton or packaging for various applications in biomedical engineering, regenerative medicine, biotechnology and more.
Deciphering the complex structure of the protein fiber was based on research work at the particle accelerators DESY (Germany) and ESRF (France) and at Technion centers: the Center for Structural Biology at the Technion, the Laurie Lockey Interdisciplinary Center for Life Sciences and Engineering, the Center for Electron Microscopy (MICA) ) and the Russell Berrie Electron Microscopy Center of Soft Matter. The research was supported by the National Science Foundation (ISF), the Ministry of Science and Technology and the Israel-US Cooperation Fund (BSF).
Recently, the Israel Society for Microscopy announced that it will award the Margolis Prize for 2020 to doctoral student Yitzhak Engelberg, due to the research breakthrough described in the present article. The award committee stated that the discovery is an "impressive achievement in the study of the structure of a human antimicrobial peptide" and that it is expected to lead to diverse applications in biotechnology, nanotechnology, the production of antibacterial drugs, tissue restoration and more.

One response
Who engineered it that way?
Strange... it's a work of art