Researchers from the Faculty of Biology at the Technion discovered that the inhibition of the protein controls the suicide of stem cells in the intestine and the function of the small and large intestine. The discovery could in the future pave new ways to treat and prevent intestinal diseases, as well as to reduce the side effects of anti-cancer treatments
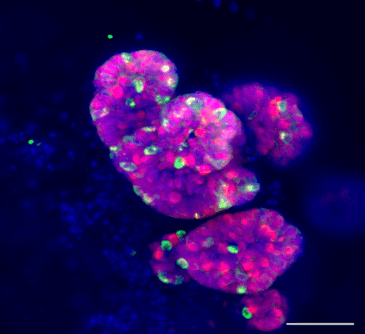
Researchers from the Faculty of Biology at the Technion located a protein responsible for critical processes in intestinal diseases. The research is expected to pave new ways in the treatment of these diseases and reduce the side effects of anti-cancer treatments.
The research published in the journal Nature Communications was led by Associate Professor Yaron Fox and PhD student Eleanor Koren from the Laboratory for Stem Cell Biology and Regenerative Medicine in the Faculty of Biology and the Lori Lockey Institute for Life Sciences and Engineering. According to the research findings, the inhibition of a protein called ARTS protects the stem cells that are essential for intestinal function.
The intestine is a tissue that regenerates very quickly, more than any other tissue in the body; The outer layer of the skin, the epithelium, is replaced every week. The small intestine is a coiled tube with an average length of 8-6 meters and it fills most of the abdominal cavity. The small intestine plays a critical role in the digestion of food and its absorption into the bloodstream and lymph, and its efficiency is related to its unique structure. The small intestine increases its surface area through finger-like protrusions called villi and made of epithelial cells that surround blood vessels and lymph. The stem cells of the small intestine are found in the intestinal crypts (crypts of Lieberkühn) which are the folds between those protrusions at the base of the intestine. The cells are constantly dividing, differentiating and forming different epithelial cells along the villi. Stem cells are relatively rare cells, responsible for the regeneration of various tissues such as skin, intestines and blood vessels. Many studies have been done so far on the subject of the self-renewal of the stem cells and their differentiation, but the mechanism of planned suicide (apoptosis) in these cells has hardly been studied. This is because for many years the stem cells were considered "immortal" cells that live until the end of the life of the organism.
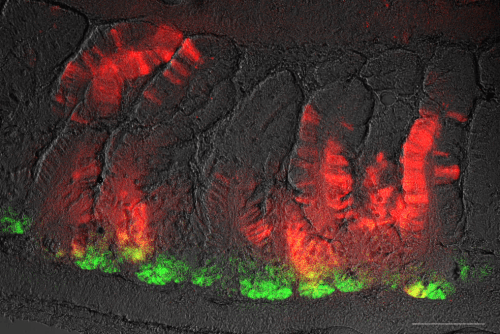
The current study deals with the suicide of the stem cells and the dramatic effect of this process on tissue regeneration and the recovery processes in the intestine. Associate Professor Yaron Fox and PhD student Eleanor Koren assumed that due to the long lifespan of the stem cells in the intestine, they may accumulate mutations and cause various diseases such as cancer. Therefore, even though they are more resistant than "normal" cells, they should be subject to a control mechanism that eliminates them in a targeted manner in the event that a mutation develops in them. The current study revealed that the stem cells in the intestine are indeed subject to such a process of programmed death. The researchers discovered that a critical protein in this process is ARTS, which is expressed in a targeted manner in the crypts of the mouse and human intestines. The research team discovered that deletion of the gene responsible for the production of said protein causes a dramatic increase in the number of stem cells in the intestine and in the size of the niche that contains them. In order to confirm their results, the researchers isolated stem cells from the mice and managed to create an "intestine in a dish" - a laboratory mini-organ (organoid) that ideally imitates the intestine in a complete animal and can also be transplanted into the animal as an organ that will function like a normal intestine. The researchers found in these organoids that the deletion of ARTS causes a significant increase in the number of stem cells and the size of the intestine. This is because some of the stem cells that were supposed to commit suicide did not go through this process.
The population of stem cells in the intestine tends to be severely damaged when a person receives anti-cancer treatments. This is one of the reasons for the formation of side effects such as fatigue, nausea, vomiting and diarrhea. The researchers discovered that in the absence of the ARTS protein, these treatments hardly harmed the stem cells. Furthermore, they found that in ARTS-deficient mice, intestinal diseases such as colitis do not manifest as severe symptoms as in normal patients. The researchers estimate that the research will pave new ways to treat and prevent intestinal diseases, as well as to reduce the side effects of anti-cancer treatments. This is through specific drugs that will inhibit ARTS in a targeted manner.
Associate Professor Yaron Fuchs is the head of the Stem Cell and Regenerative Medicine Laboratory, a faculty member in the Faculty of Biology and a member of the Lori Loki Center for Engineering and Life Sciences and the Integrated Center for Cancer Research at the Technion. The laboratory team under his leadership focuses on isolating new populations of stem cells, researching the suicide mechanism of these special cells and promoting new techniques for regenerative medicine and the treatment of cancer.
The present study was conducted with the support of the Ministry of Science Foundation (Kamin), ICRF RCDA and GIF.
