Understanding the mechanism of action may affect the treatments for cancer and hyperpigmentation
Researchers at the Technion "caught the activity of the enzyme tyrosinase red-handed at the molecular level, using advanced X-ray crystallography equipment. This is what the journal Nature Communications reports this week.
Two technical faculties are involved in the research: the Faculty of Biotechnology and Food Engineering and the Shulich Faculty of Chemistry. In the laboratory of Prof. Eilat Fishman, in the Faculty of Biotechnology and Food Engineering, the focus is on understanding the relationship between the structures of enzymes and their function. One of these enzymes is tyrosinase, which is common at all levels of life: in microorganisms, plants and animals. It is responsible for the first steps in the production of the dark pigment melanin. In the human body, tyrosinase is found in melanocyte cells in the epidermis, and the melanin produced not only determines the color of the skin, hair and retina, but also plays an important role in protection against UV radiation. A mutation in this enzyme is the main cause of albinism (whiteness), leading to visual disturbances and sensitivity to radiation. In fruits and vegetables, the tyrosinase activity causes browning, and the result is economic damage to the growers.
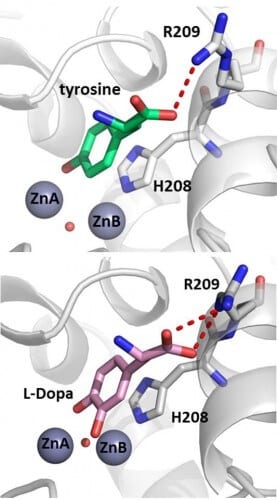
The tyrosinase performs two successive oxidation reactions: the conversion of small organic molecules (such as the amino acid tyrosine) into di-phenol, followed by the conversion of the di-phenol into quinone, which enables the formation of melanin. The enzyme needs two copper ions, and the accepted opinion in the scientific community was that each of the two reactions takes place on a different copper ion.
Mor Goldfeder, a doctoral student under joint supervision with Professor Noam Adir from the Shulich Faculty of Chemistry, and postdoctoral student Dr. Rita Cantiv, used X-ray crystallography to solve for the first time the structure of bacterial tyrosinase in the presence of the two substrates: mono-phenol tyrosine, and diphenol dopa (L-Dopa). In addition, they were able to solve a structure with an additional substrate, where the catalytic product of the enzyme could be clearly seen during its formation. Doctoral student Sivan Issachar-Ovadat, who researches uses of the enzyme in food, was a partner in the research. The results prove that all the substrates are stabilized in exactly the same way in the active site of the enzyme, and face only one of the copper ions.
Understanding the mechanism of action and the structure of tyrosinase may help in the treatment of hyperpigmentation and age spots, in improving the sensitivity of melanoma cells to radiation therapy, and in preventing browning in fruits and vegetables.
